Abstract
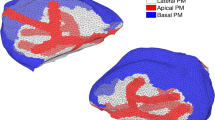
We construct a three-dimensional anatomically accurate multicellular model of a parotid gland acinus to investigate the influence that the topology of its lumen has on primary fluid secretion. Our model consists of seven individual cells, coupled via a common lumen and intercellular signalling. Each cell is equipped with the intracellular calcium (\(\mathrm{Ca}^{2+}\))-signalling model developed by Pages et al, Bull Math Biol 81: 1394–1426, 2019. https://doi.org/10.1007/s11538-018-00563-z and the secretion model constructed by Vera-Sigüenza et al., Bull Math Biol 81: 699–721, 2019. https://doi.org/10.1007/s11538-018-0534-z. The work presented here is a continuation of these studies. While previous mathematical research has proven invaluable, to the best of our knowledge, a multicellular modelling approach has never been implemented. Studies have hypothesised the need for a multiscale model to understand the primary secretion process, as acinar cells do not operate on an individual basis. Instead, they form racemous clusters that form intricate water and protein delivery networks that join the acini with the gland’s ducts-questions regarding the extent to which the acinus topology influences the efficiency of primary fluid secretion to persist. We found that (1) The topology of the acinus has almost no effect on fluid secretion. (2) A multicellular spatial model of secretion is not necessary when modelling fluid flow. Although the inclusion of intercellular signalling introduces vastly more complex dynamics, the total secretory rate remains fundamentally unchanged. (3) To obtain an acinus, or better yet a gland flow rate estimate, one can multiply the output of a well-stirred single-cell model by the total number of cells required.













Similar content being viewed by others
Notes
For results in cells 1 to 5, refer to the supplementary material accompanying this article.
References
Ambudkar IS (2014) Ca2+ signaling and regulation of fluid secretion in salivary gland acinar cells. Cell Calcium 55(6):297–305
Barbe AG, Schmidt P, Bussmann M, Kunter H, Noack MJ, Röhrig G (2018) Xerostomia and hyposalivation in orthogeriatric patients with fall history and impact on oral health-related quality of life. Clin Interv Aging 13:1971
Berridge MJ, Galione A (1988) Cytosolic calcium oscillators. FASEB J 2(15):3074–3082
Boitano S, Dirksen ER, Sanderson MJ (1992) Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science 258(5080):292–295
Evans RL, Park K, Turner RJ, Watson GE, Nguyen H-V, Dennett MR, Hand AR, Flagella M, Shull GE, Melvin JE (2000) Severe impairment of salivation in Na\(^{+}\)/K\(^{+}\)/2Cl\(^{-}\) cotransporter (NKCC1)-deficient mice. J Biol Chem 275(35):26,720–26,726. https://doi.org/10.1074/jbc.M003753200
Ewert P, Aguilera S, Alliende C, Kwon Y-J, Albornoz A, Molina C, Urzúa U, Quest AF, Olea N, Pérez P et al (2010) Disruption of tight junction structure in salivary glands from sjögren’s syndrome patients is linked to proinflammatory cytokine exposure. Arthr Rheum 62(5):1280–1289
Foskett JK, Melvin JE (1989) Activation of salivary secretion: coupling of cell volume and [Ca\(^{2+}\)]\(_i\) in single cells. Science 244(4912):1582–1585
Gin E, Crampin EJ, Brown DA, Shuttleworth TJ, Yule DI, Sneyd J (2007) A mathematical model of fluid secretion from a parotid acinar cell. J Theor Biol 248(1):64–80. https://doi.org/10.1016/j.jtbi.2007.04.021
Giovannucci DR, Bruce JI, Straub SV, Arreola J, Sneyd J, Shuttleworth TJ, Yule DI (2002) Cytosolic Ca\(^{2+}\) and Ca\(^{2+}\)-activated Cl\(^-\) current dynamics: insights from two functionally distinct mouse exocrine cells. J Physiol 540(2):469–484
Höfer T, Politi A, Heinrich R (2001) Intercellular Ca\(^{2+}\) wave propagation through gap-junctional Ca\(^{2+}\) diffusion: a theoretical study. Biophys J 80(1):75–87
Holmberg KV, Hoffman MP (2014) Anatomy, biogenesis and regeneration of salivary glands. In: Saliva: secretion and functions, vol 24, Karger Publishers, pp 1–13
Inagaki T, Ono K, Masuda W, Iida T, Hosokawa R, Inenaga K (2010) Differences in the Ca\(^{2+}\) response resulting from neurotransmitter stimulations of rat parotid acini and ducts. Auton Neurosci 154(1–2):102–107
Larina O, Thorn P (2005) Ca2+ dynamics in salivary acinar cells: distinct morphology of the acinar lumen underlies near-synchronous global ca2+ responses. J Cell Sci 118(18):4131–4139
Maclaren OJ, Sneyd J, Crampin EJ (2012) Efficiency of primary saliva secretion: an analysis of parameter dependence in dynamic single-cell and acinus models, with application to aquaporin knockout studies. J Membr Biol 245(1):29–50. https://doi.org/10.1007/s00232-011-9413-3
Maclaren OJ, Sneyd J, Crampin EJ (2013) What do aquaporin knockout studies tell us about fluid transport in epithelia? J Membr Biol 246(4):297–305
Mangos J, McSherry NR, Nousia-Arvanitakis S, Irwin K (1973) Secretion and transductal fluxes of ions in exocrine glands of the mouse. Am J Physiol 225(1):18–24. https://doi.org/10.1152/ajplegacy.1973.225.1.18
Martin C, Young J (1971) Electrolyte concentrations in primary and final saliva of the rat sublingual gland studied by micropuncture and catheterization techniques. Pflügers Archiv 324(4):344–360
Melvin JE, Yule D, Shuttleworth T, Begenisich T (2005) Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol 67:445–469. https://doi.org/10.1146/annurev.physiol.67.041703.084745
Nagato T, Tandler B (1986) Gap junctions in rat sublingual gland. Anat. Rec 214(1):71–75
Nauntofte B (1992) Regulation of electrolyte and fluid secretion in salivary acinar cells. Am J Physiol 263(6):G823–G837. https://doi.org/10.1152/ajpgi.1992.263.6.G823
Pages N, Vera-Sigüenza E, Rugis J, Kirk V, Yule DI, Sneyd J (2019) A model of Ca\(^{2+}\) dynamics in an accurate reconstruction of parotid acinar cells. Bull Math Biol 5:1394–1426. https://doi.org/10.1007/s11538-018-00563-z
Palk L, Sneyd J, Shuttleworth TJ, Yule DI, Crampin EJ (2010) A dynamic model of saliva secretion. J Theor Biol 266(4):625–640. https://doi.org/10.1016/j.jtbi.2010.06.027
Palk L, Sneyd J, Patterson K, Shuttleworth TJ, Yule DI, Maclaren O, Crampin EJ (2012) Modelling the effects of calcium waves and oscillations on saliva secretion. J Theor Biol 305:45–53. https://doi.org/10.1016/j.jtbi.2012.04.009
Peña-Münzenmayer G, Catalán MA, Kondo Y, Jaramillo Y, Liu F, Shull GE, Melvin JE (2015) Ae4 (Slc4a9) anion exchanger drives Cl\(^-\)uptake-dependent fluid secretion by mouse submandibular gland acinar cells. J Biol Chem 290(17):10677–10688. https://doi.org/10.1074/jbc.M114.612895
Proctor GB (2000) (2016) The physiology of salivary secretion. Periodontology 70(1):11–25
Riva A, Riva-Testa F (1973) Fine structure of acinar cells of human parotid gland. Anat Rec 176(2):149–165
Saez JC, Connor JA, Spray DC, Bennett M (1989) Hepatocyte gap junctions are permeable to the second messenger, inositol 1, 4, 5-trisphosphate, and to calcium ions. Proc Nat Acad Sci 86(8):2708–2712
Sanderson MJ (1995) Intercellular calcium waves mediated by inositol trisphosphate. Ciba Found Symp 188:175–189
Scully C (2003) Drug effects on salivary glands: dry mouth. Oral Dis 9(4):165–176
Sigüenza EA, Catalàn M, Peña-Münzenmayer G, Melvin E, Sneyd J (2018) A mathematical model supports a key role for ae4 (slc4a9) in salivary gland secretion. Bull Math Biol 80(2):255–282. https://doi.org/10.1007/s11538-017-0370-6
Sigüenza E, Pages N, Rugis J, Yule DI, Sneyd J (2019) A mathematical model of fluid transport in an accurate reconstruction of parotid acinar cells. Bull Math Biol 81(3):699–721. https://doi.org/10.1007/s11538-018-0534-z
Silva P, Stoff J, Field M, Fine L, Forrest J, Epstein F (1977) Mechanism of active chloride secretion by shark rectal gland: role of Na–K–ATPase in chloride transport. Am J Physiol 233(4):F298–F306. https://doi.org/10.1038/ki.1996.224
Sneyd J, Wilkins M, Strahonja A, Sanderson MJ (1998) Calcium waves and oscillations driven by an intercellular gradient of inositol (1, 4, 5)-trisphosphate. Biophys Chem 72(1–2):101–109
Sneyd J, Tsaneva-Atanasova K, Bruce J, Straub S, Giovannucci D, Yule D (2003) A model of calcium waves in pancreatic and parotid acinar cells. Biophys J 85(3):1392–1405
Sneyd J, Means S, Zhu M, Hak-Won J, Yule D (2017) Modeling calcium waves in an anatomically accurate three-dimensional parotid acinar cell. J Theor Biol 419:383–393
Tanimura A (2009) Mechanism of calcium waves and oscillations in non-excitable cells. Int J Dent Oral-Med Sci 8(1):1–11. https://doi.org/10.5466/ijoms.8.1
Tanimura A, Matsumoto Y, Tojyo Y (1998) Polarized Ca\(^{2+}\) release in saponin-permeabilized parotid acinar cells evoked by flash photolysis of ‘cagedâǍŹ inositol 1,4,5-trisphosphate. Biochem J 332(3):769–772
Wang T, Montell C (2006) A phosphoinositide synthase required for a sustained light response. J Neurosci 26(49):12,816–12,825
Acknowledgements
This work was supported by the U.S. National Institutes of Health (NIDCR) Grant RO1DE019245-10 (ES, DY, and JS) and the Marsden Fund of the Royal Society of New Zealand Grant 3708441 (NP and JS). High-performance computing facilities and support were provided by the New Zealand eScience Infrastructure (NeSI). Funded jointly by NeSI’s collaborator institutions and through the New Zealand Ministry of Business, Innovation and Employment’s Research Infrastructure programme (ES, NP, JS, and JR). Finally, we would like to thank NVIDIA Corporation for a K40 GPU Grant (JR).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix
Fluxes of the Model
i. Sodium/Potassium ATPases (NaK-ATPases–Basolateral)
Membrane surface density: \(\alpha _{\text {NaK}} = 1.6\) (amol/\(\upmu \hbox {m}^2\))
NaK-ATPase rate: \(r=0.0016\) (mM\(^{-3}\hbox { s}^{-1}\))
ii. Sodium–Potassium–Chloride Cotransporters (Nkcc1 - Basolateral)
Membrane surface density: \(\alpha _{\text {Nkcc1}} = 2.15\) (amol/\(\upmu \)m\(^2\))
Nkcc1 rate: \(a_1 = 157.55\) (s\(^{-2}\))
Nkcc1 rate: \(a_2 = 2.009 \times 10^{-5}\) (mM\(^{-4}\) s\(^{-2}\))
Nkcc1 rate: \(a_3 = 1.0306\) (s\(^{-1}\))
Nkcc1 rate: \(a_4 = 1.38 \times 10^{-6}\) (mM\(^{-4}\) s\(^{-1}\))
iii. Sodium/Proton Antiporter (Nhe1–Basolateral)
Membrane surface density: \(G_{\text {Nhe1}} = 0.9\) (amol/\(\upmu \)m\(^2\))
Half maximal \(\mathrm{Na}^+\) concentration: K\(_{\text {Na}}=15\) (mM)
Half maximal \(\mathrm{H}^+\) concentration: K\(_{\text {H}}=4.5 \times 10^{-4}\) (mM)
iv. Sodium-Bicarbonate/Chloride Exchanger (Ae4–Basolateral)
Membrane surface density: \(G_{\text {Ae4}} = 1.3\) (amol/\(\upmu \hbox {m}^2\))
Half maximal \(\mathrm{Na}^+\) concentration: \(\hbox {K}_{\text {Na}}=15\) (mM)
Half maximal \(\mathrm{H}^+\) concentration: \(\hbox {K}_{\text {B}}=1\times 10^{4}\) (mM)
Half maximal \(\mathrm{H}^+\) concentration: \(\hbox {K}_{\text {Cl}}=5.6\) (mM)
v. Intracellular Bicarbonate Buffering (CA-IV)
Buffering association rate: \(k_\mathrm{p}=312\) (s\(^{-1}\))
Buffering dissociation rate: \(k_n=0.132\) (s\(^{-1}\))
vi. Basolateral Calcium-Activated Potassium Channels (K.Ca1 - BK/IK)
Half maximal concentration: \(K_{K_{\text {Ca}}}=0.3\) (\(\upmu \)M)
Hill coefficient: \(\eta _2=4.7\)
\(\mathrm{K}^+\) channel conductance: \(G_{\text {K}}=14.6\) (nS)
vii. Tight Junctional Ionic Fluxes (Paracellular)
Temperature: \(T=310\) (Kelvin)
Universal Gas Constant: \(R=8.314\) (J mol\(^{-1}\) K\(^{-1}\))
Faraday’s Constant: \(F=6485.332\) (C)
Tight Junctional \(\mathrm{Na}^+\) conductance: \(G^t_{\text {Na}}=0.2\) (nS)
Tight Junctional \(\mathrm{K}^+\) conductance: \(G^t_{\text {K}}=0.16\) (nS)
viii. Apical Calcium-Activated Chloride Channels (TMeM16a - KCa)
Half maximal concentration: \(K_{Cl_{\text {Ca}}}=0.2\) (\(\upmu \)M)
Hill coefficient: \(\eta _1=4.49\)
\(\mathrm{K}^+\) channel conductance: \(G_{\text {Cl}}=18.2\) (nS)
Model Summary
1.1 Fluid Flow Model
For any cell \(c = 1,\ldots ,7\), and its neighbours n,
Here, the expression: \(\sum _* j^*_w [\mathrm ion]^*\) represents the sum of all neighbouring luminal convective water fluxes into the luminal segment shared between cells c and n. Similarly, \(\sum _* j^*_w\) represents the sum of all neighbouring luminal water fluxes in and out to the luminal segment shared between cells c and n.
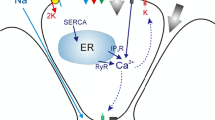
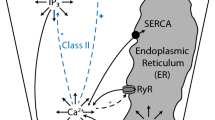
1.2 Calcium Dynamics Model
i. Inositol-1,4,5-Trisphosphate Receptors (InsP\(_3\)R)
ii. Sarcoplasmic/Endoplasmic Ca2+ ATPase Pumps (SERCA)
iii. Inositol-1,4,5-Trisphosphate (InsP\(_3\) Dynamics)
iv. Wave Propagation Model
v. Equations of the Ca\(^{2+}\) Model
v. Boundary Conditions of the Ca\(^{2+}\) Model
Parameter | Description | Value | Units |
|---|---|---|---|
\(V_{\mathrm{Wav}}^{\mathrm{max}}\) | Maximal rate of the wave propagation model | 0.025 | \(\upmu \)M s\(^{-1}\) |
\(K_{\mathrm{wav1}}\) | Half maximal concentration of calcium for positive feedback on wave propagation | 0.2 | \(\upmu \)M |
\(d_{\mathrm{Wav}}\) | Critical distance for the expression of the wave propagation | 1 | \(\upmu \)m |
\(K_{\mathrm{hWav}}\) | Half maximal concentration of calcium for negative feedback on wave propagation | 0.15 | \(\upmu \)M |
\(\tau _{\mathrm{wav}}\) | Time scaling for negative feedback of calcium on the wave propagation | 10 | s |
\(k_\mathrm{IPR}\) | IPR rate | 30 | \(\upmu \,\hbox {M}\,\mathrm{m}\,\mathrm{s}^{-1}\) |
\(k_{\beta }\) | – | 0.4 | – |
\(K_c\) | Half maximal concentration of calcium for positive feedback on IPR | 0.2 | \(\upmu \)M |
\(K_p\) | Half maximal concentration of \(\mathrm{InsP}_3\) for feedback on the IPR | 0.2 | \(\upmu \)M |
\(K_h\) | Half maximal concentration of calcium for negative feedback on IPR | 0.08 | \(\upmu \)M |
\(K_{\tau }\) | Half maximal concentration of calcium for feedback on the time scaling factor | 0.1 | \(\upmu \)M |
\(\tau _{\max }\) | Time scaling for negative feedback of calcium on the IPR | 1000 | s |
\(K_\mathrm{PLC}\) | Half maximal concentration of \(\mathrm{Ca}^{2+}\) for feedback on PLC | 0.07 | \(\upmu \)M |
\(K_{3K}\) | Half maximal concentration of \(\mathrm{Ca}^{2+}\) for feedback on Insp-3-kinase | 0.4 | \(\upmu \)M |
\(V_{3K}\) | \(\mathrm{InsP}_3\) metabolisation rate by InsP-3-kinase | 0.05 | s\(^{-1}\) |
\(V_{5K}\) | \(\mathrm{InsP}_3\) degradation rate by InsP-5-phosphatase | 0.05 | s\(^{-1}\) |
\(\hbox {d}_\mathrm{PLC}\) | Critical distance from the basal membrane for the expression of PLC | 0.8 | \(\upmu \)m |
\(\hbox {dl}_\mathrm{PLC}\) | Minimum distance from the apical membrane for the expression of PLC | 0.6 | \(\upmu \)m |
\(k_\mathrm{PLC}\) | Maximum PLC rate | 0.6 | \(\upmu \)M s\(^{-1}\) |
\(d_b(x)\) | Distance to the basal region of the point x | – | \(\upmu \)m |
\(d_\mathrm{a}(x)\) | Distance to the basal region of the point x | – | \(\upmu \)m |
\(D_c\) | Calcium diffusion | 5 | \(\upmu \)m\(^2\) s\(^{-1}\) |
\(D_p\) | IP\(_3\) diffusion coefficient | 285 | \(\upmu \)m\(^2\) s\(^{-1}\) |
\(\gamma \) | Ratio ER volume/cytosolic volume | 0.185 | – |
\(K_{K_{\text {Ca}}}\) | Half maximal concentration | 0.3 | \(\upmu \)M |
\(\eta _2\) | Hill coefficient | 4.7 | – |
Parameter | Description | Value | Units |
|---|---|---|---|
\(G_{\text {K}}\) | \(\mathrm{Cl}^-\) channel conductance | 14.6 | nS |
\(K_{Cl_{\text {Ca}}}\) | Half maximal concentration | 0.2 | \(\upmu \)M |
\(\eta \) | Hill coefficient | 4.49 | – |
\(G_{\text {Cl}}\) | \(\mathrm{Cl}^-\) channel conductance | 18.2 | nS |
\(V_{\mathrm{Wav}}^{\mathrm{max}}\) | Wave propagation rate | 0.025 | \(\upmu \)M s\(^{-1}\) |
\(K_{\mathrm{wav1}}\) | Half maximal concentration of calcium | 0.2 | \(\upmu \)M |
\(d_{\mathrm{Wav}}\) | Critical distance of wave propagation | 1 | \(\upmu \)m |
\(K_{\mathrm{hWav}}\) | Half maximal concentration of calcium propagation | 0.15 | \(\upmu \)M |
\(\tau _{\mathrm{wav}}\) | Time scaling | 10 | s |
\(S_b^{\{1\}}\) | Cell 1 Basolateral Surface Area | 285.0 | \(\upmu \hbox {m}^2\) |
\(S_b^{\{2\}}\) | Cell 2 Basolateral Surface Area | 238.6 | \(\upmu \hbox {m}^2\) |
\(S_b^{\{3\}}\) | Cell 3 Basolateral Surface Area | 311.9 | \(\upmu \hbox {m}^2\) |
\(S_b^{\{4\}}\) | Cell 4 Basolateral Surface Area | 284.8 | \(\upmu \hbox {m}^2\) |
\(S_b^{\{5\}}\) | Cell 5 Basolateral Surface Area | 144.03 | \(\upmu \hbox {m}^2\) |
\(S_b^{\{6\}}\) | Cell 6 Basolateral Surface Area | 304.05 | \(\upmu \hbox {m}^2\) |
\(S_b^{\{7\}}\) | Cell 7 Basolateral Surface Area | 324.8 | \(\upmu \hbox {m}^2\) |
\(S_\mathrm{a}^{\{1\}}\) | Cell 1 Apical Surface Area | 92.7 | \(\upmu \hbox {m}^2\) |
\(S_\mathrm{a}^{\{2\}}\) | Cell 2 Apical Surface Area | 100.4 | \(\upmu \hbox {m}^2\) |
\(S_\mathrm{a}^{\{3\}}\) | Cell 3 Apical Surface Area | 93.9 | \(\upmu \hbox {m}^2\) |
\(S_\mathrm{a}^{\{4\}}\) | Cell 4 Apical Surface Area | 95.3 | \(\upmu \hbox {m}^2\) |
\(S_\mathrm{a}^{\{5\}}\) | Cell 5 Apical Surface Area | 48.9 | \(\upmu \hbox {m}^2\) |
\(S_\mathrm{a}^{\{6\}}\) | Cell 6 Apical Surface Area | 73.9 | \(\upmu \hbox {m}^2\) |
\(S_\mathrm{a}^{\{7\}}\) | Cell 7 Apical Surface Area | 54.3 | \(\upmu \hbox {m}^2\) |
\(\omega _i^{\{1\}}\) | Cell 1 Resting Volume | 1004.8 | \(\upmu \hbox {m}^3\) |
\(\omega _i^{\{2\}}\) | Cell 2 Resting Volume | 1006.9 | \(\upmu \hbox {m}^3\) |
\(\omega _i^{\{3\}}\) | Cell 3 Resting Volume | 1084.8 | \(\upmu \hbox {m}^3\) |
\(\omega _i^{\{4\}}\) | Cell 4 Resting Volume | 1109.3 | \(\upmu \hbox {m}^3\) |
\(\omega _i^{\{5\}}\) | Cell 5 Resting Volume | 495.04 | \(\upmu \hbox {m}^3\) |
\(\omega _i^{\{6\}}\) | Cell 6 Resting Volume | 1036.2 | \(\upmu \hbox {m}^3\) |
\(\omega _i^{\{7\}}\) | Cell 7 Resting Volume | 904.2 | \(\upmu \hbox {m}^3\) |
Rights and permissions
About this article
Cite this article
Vera-Sigüenza, E., Pages, N., Rugis, J. et al. A Multicellular Model of Primary Saliva Secretion in the Parotid Gland. Bull Math Biol 82, 38 (2020). https://doi.org/10.1007/s11538-020-00712-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11538-020-00712-3