Abstract
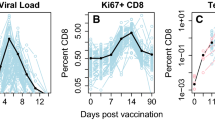
Does target cell depletion, innate immunity, or adaptive immunity play the dominant role in controlling primary acute viral infections? Why do some individuals have higher peak virus titers than others? Answering these questions is a basic problem in immunology and can be particularly difficult in humans due to limited data, heterogeneity in responses in different individuals, and limited ability for experimental manipulation. We address these questions for infections following vaccination with the live attenuated yellow fever virus (YFV-17D) by analyzing viral load data from 80 volunteers. Using a mixed effects modeling approach, we find that target cell depletion models do not fit the data as well as innate or adaptive immunity models. Examination of the fits of the innate and adaptive immunity models to the data allows us to select a minimal model that gives improved fits by widely used model selection criteria (AICc and BIC) and explains why it is hard to distinguish between the innate and adaptive immunity models. We then ask why some individuals have over 1000-fold higher virus titers than others and find that most of the variation arises from differences in the initial/maximum growth rate of the virus in different individuals.






Similar content being viewed by others
References
Akondy RS, Johnson PL, Nakaya HI, Edupuganti S, Mulligan MJ, Lawson B, Miller JD, Pulendran B, Antia R, Ahmed R (2015) Initial viral load determines the magnitude of the human CD8 T cell response to yellow fever vaccination. PNAS 112(10):3050–3055
Antia R, Koella JC (1994) A model of non-specific immunity. J Theor Biol 168(2):141–150
Antia R, Levin BR, May RM (1994) Within-host population dynamics and the evolution and maintenance of microparasite virulence. Am Nat 144(3):457–472
Antia R, Yates A, de Roode JC (2008) The dynamics of acute malaria infections. i. effect of the parasite’s red blood cell preference. Proc Biol Sci 275(1641):1449–58. https://doi.org/10.1098/rspb.2008.0198
Baccam P, Beauchemin C, Macken CA, Hayden FG, Perelson AS (2006) Kinetics of influenza a virus infection in humans. J Virol 80(15):7590–7599
Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, Miller VL, Virgin HW (2007) Herpesvirus latency confers symbiotic protection from bacterial infection. Nature 447(7142):326–329
Beauchemin CA, Handel A (2011) A review of mathematical models of influenza A infections within a host or cell culture: lessons learned and challenges ahead. BMC Public Health 11(Suppl 1):S7
Ben-Shachar R, Koelle K (2015) Minimal within-host dengue models highlight the specific roles of the immune response in primary and secondary dengue infections. J R Soc Interface 12(103):20140,886
Best K, Guedj J, Madelain V, de Lamballerie X, Lim SY, Osuna CE, Whitney JB, Perelson AS (2017) Zika plasma viral dynamics in nonhuman primates provides insights into early infection and antiviral strategies. PNAS 114(33):8847–8852
Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, Thompson EA, Fraser KA, Rosato PC, Filali-Mouhim A et al (2016) Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532(7600):512–516
Canini L, Carrat F (2011) Population modeling of influenza A/H1N1 virus kinetics and symptom dynamics. J Virol 85(6):2764–2770
Carr EJ, Dooley J, Garcia-Perez JE, Lagou V, Lee JC, Wouters C, Meyts I, Goris A, Boeckxstaens G, Linterman MA, Liston A (2016) The cellular composition of the human immune system is shaped by age and cohabitation. Nat Immunol 17(4):461–468
Chamberlin TC (1890) The method of multiple working hypotheses: with this method the dangers of parental affection for a favorite theory can be circumvented. Science 15:92–96. https://doi.org/10.1126/science.148.3671.754
Davis MM (2008) A prescription for human immunology. Immunity 29(6):835–838
Edupuganti S, Eidex RB, Keyserling H, Akondy RS, Lanciotti R, Orenstein W, del Rio C, Pan Y, Querec T, Lipman H, Barrett A, Ahmed R, Teuwen D, Cetron M, Mulligan MJ (2013) A randomized, double-blind, controlled trial of the 17D yellow fever virus vaccine given in combination with immune globulin or placebo: comparative viremia and immunogenicity. Am J Trop Med Hyg 88(1):172–177
Elemans M, Thiébaut R, Kaur A, Asquith B (2011) Quantification of the relative importance of CTL, B cell, NK cell, and target cell limitation in the control of primary SIV-infection. PLOS Comput Biol 7(3):e1001,103
Guedj J, Thiébaut R, Commenges D (2007) Maximum likelihood estimation in dynamical models of HIV. Biometrics 63(4):1198–1206
Handel A, Longini IM, Antia R (2010) Towards a quantitative understanding of the within-host dynamics of influenza A infections. J R Soc Interface 7(42):35–47
Handel A, Longini IM Jr, Antia R (2007) Neuraminidase inhibitor resistance in influenza: assessing the danger of its generation and spread. PLoS Comput Biol 3(12):e240. https://doi.org/10.1371/journal.pcbi.0030240
Handel A, Longini IM Jr, Antia R (2010) Towards a quantitative understanding of the within-host dynamics of influenza a infections. J R Soc Interface 7(42):35–47. https://doi.org/10.1098/rsif.2009.0067
Haydon DT, Matthews L, Timms R, Colegrave N (2003) Top-down or bottom-up regulation of intra-host blood-stage malaria: do malaria parasites most resemble the dynamics of prey or predator? Proc Biol Sci 270(1512):289–98. https://doi.org/10.1098/rspb.2002.2203
Hilborn R, Mangel M (1997) The ecological detective: confronting models with data. Monographs in population biology 28. Princeton University Press, Princeton
Kochin BF, Yates AJ, de Roode JC, Antia R (2010) On the control of acute rodent malaria infections by innate immunity. PLoS One 5(5):e10,444. https://doi.org/10.1371/journal.pone.0010444
Kuhn E, Lavielle M (2005) Maximum likelihood estimation in nonlinear mixed effects models. Comput Stat Data Anal 49(4):1020–1038
Le D, Miller JD, Ganusov VV (2015) Mathematical modeling provides kinetic details of the human immune response to vaccination. Front Cell Infect Microbiol 4:1–13
Li Y, Handel A (2014) Modeling inoculum dose dependent patterns of acute virus infections. J Theor Biol 347:63–73
Lindstrom MJ, Bates DM (1990) Nonlinear mixed effects models for repeated measures data. Biometrics 46(3):673–687
McQueen PG, McKenzie FE (2004) Age-structured red blood cell susceptibility and the dynamics of malaria infections. PNAS 101(24):9161–9166
Miao H, Hollenbaugh JA, Zand MS, Holden-Wiltse J, Mosmann TR, Perelson AS, Wu H, Topham DJ (2010) Quantifying the early immune response and adaptive immune response kinetics in mice infected with influenza A virus. J Virol 84(13):6687–6698
Mideo N, Savill NJ, Chadwick W, Schneider P, Read AF, Day T, Reece SE (2011) Causes of variation in malaria infection dynamics: insights from theory and data. Am Nat 178(6):E174–88. https://doi.org/10.1086/662670
Monath T, Staples J, Barrett A (2012) Yellow fever vaccine, 6th edn. Elsevier Saunders, Philadelphia, pp 870–968
Osborne LC, Monticelli LA, Nice TJ, Sutherland TE, Siracusa MC, Hepworth MR, Tomov VT, Kobuley D, Tran SV, Bittinger K, Bailey AG, Laughlin AL, Bouvher JL, Wherry EJ, Bushman DF, Allen JE, Virgin HW, Artis D (2014) Virus-helminth coinfection reveals a microbiota-independent mechanism of immunomodulation. Science 345(6196):578–582
Painter SD, Ovsyannikova IG, Poland GA (2015) The weight of obesity on the human immune response to vaccination. Vaccine 33(36):4422–4429
Pawelek KA, Huynh GT, Quinlivan M, Cullinane A, Rong L, Perelson AS (2012) Modeling within-host dynamics of influenza virus infection including immune responses. PLoS Comput Biol 8(6):e1002,588–e1002,588
Perelson AS (2002) Modelling viral and immune system dynamics. Nat Rev Immunol 2(1):28–36
Phillips AN (1996) Reduction of hiv concentration during acute infection: independence from a specific immune response. Science 271(5248):497–499
Pinheiro JC, Bates DM (1995) Approximations to the log-likelihood function in the nonlinear mixed-effects model. J. Comput. Gr. Stat. 4(1):12–35
Poland G, Ovsyannikova I, Jacobson R, Smith D (2007) Heterogeneity in vaccine immune response: the role of immunogenetics and the emerging field of vaccinomics. Clin. Pharmacol. Ther. 82(6):653–664
Rai D, Pham NLL, Harty JT, Badovinac VP (2009) Tracking the total CD8 T cell response to infection reveals substantial discordance in magnitude and kinetics between inbred and outbred hosts. J Immunol 183(12):7672–7681
Regoes RR, Antia R, Garber DA, Silvestri G, Feinberg MB, Staprans SI (2004) Roles of target cells and virus-specific cellular immunity in primary simian immunodeficiency virus infection. J Virol 78(9):4866–75
Reynolds SR, Celis E, Sette A, Oratz R, Shapiro RL, Johnston D, Fotino M, Bystryn JC (1998) Hla-independent heterogeneity of CD8+ T cell responses to MAGE-3, Melan-A/MART-1, gp100, tyrosinase, MC1R, and TRP-2 in vaccine-treated melanoma patients. J Immunol 161(12):6970–6976
Saenz RA, Quinlivan M, Elton D, MacRae S, Blunden AS, Mumford JA, Daly JM, Digard P, Cullinane A, Grenfell BT, McCauley JW, Wood JLN, Gog JR (2010) Dynamics of influenza virus infection and pathology. J Virol 84(8):3974–3983
Samson A, Lavielle M, Mentré F (2006) Extension of the saem algorithm to left-censored data in nonlinear mixed-effects model: application to HIV dynamics model. Comput Stat Data Anal 51(3):1562–1574
Smith AM, Adler FR, Ribeiro RM, Gutenkunst RN, McAuley JL, McCullers JA, Perelson AS (2013) Kinetics of coinfection with influenza A virus and streptococcus pneumoniae. PLoS Pathog 9(3):e1003,238
Smith AM, Adler FR, Ribeiro RM, Gutenkunst RN, McAuley JL, McCullers JA, Perelson AS (2013) Kinetics of coinfection with influenza a virus and streptococcus pneumoniae. PLoS Pathog 9(3):e1003,238. https://doi.org/10.1371/journal.ppat.1003238
Stafford MA, Corey L, Cao Y, Daar ES, Ho DD, Perelson AS (2000) Modeling plasma virus concentration during primary hiv infection. J Theor Biol 203(3):285–301. https://doi.org/10.1006/jtbi.2000.1076
Yap GS, Stevenson MM (1994) Blood transfusion alters the course and outcome of plasmodium chabaudi as infection in mice. Infect Immun 62(9):3761–3765
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by four NIH Grants NIH U54GM111274, NIH R01AI110720 (to R. Antia), NIH U19AI11789102 (to R.Antia and R.Ahmed), and U19AI057266 (to R. Ahmed).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moore, J., Ahmed, H., Jia, J. et al. What Controls the Acute Viral Infection Following Yellow Fever Vaccination?. Bull Math Biol 80, 46–63 (2018). https://doi.org/10.1007/s11538-017-0365-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-017-0365-3