Abstract
Background
The emergence of drug resistance is one of the most prevalent reasons for treatment failure in HIV therapy. This has severe implications for the cost of treatment, survival and quality of life.
Methods
We use mathematical modelling to describe the interaction between T cells, HIV-1 and protease inhibitors. We use impulsive differential equations to examine the effects of different levels of protease inhibitors in a T cell. We classify three different regimes according to whether the drug efficacy is low, intermediate or high. The model includes two strains: the wild-type strain, which initially dominates in the absence of drugs, and the mutant strain, which is the less efficient competitor, but has more resistance to the drugs.
Results
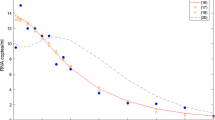
Drug regimes may take trajectories through one, two or all three regimes, depending on the dosage and the dosing schedule. Stability analysis shows that resistance does not emerge at low drug levels. At intermediate drug levels, drug resistance is guaranteed to emerge. At high drug levels, either the drug-resistant strain will dominate or, in the absence of longer-lived reservoirs of infected cells, a region exists where viral elimination could theoretically occur. We provide estimates of a range of dosages and dosing schedules where the trajectories lie either solely within a region or cross multiple regions.
Conclusion
Under specific circumstances, if the drug level is physiologically tolerable, elimination of free virus is theoretically possible. This forms the basis for theoretical control using combination therapy and for understanding the effects of partial adherence.









Similar content being viewed by others
References
Allen, L. J. S. (2006). Introduction to mathematical biology (pp. 150–151). Upper Saddle River: Pearson Education.
Bainov, D. D., & Simeonov, P. S. (1989). Systems with impulsive effect. Chichester: Ellis Horwood Ltd.
Bainov, D. D., & Simeonov, P. S. (1993). Impulsive differential equations: periodic solutions and applications. Burnt Mill: Longman Scientific and Technical.
Bainov, D. D., & Simeonov, P. S. (1995). Impulsive differential equations: asymptotic properties of the solutions. Singapore: World Scientific.
Bhunu, C. P., Garira, W., & Magombedze, G. (2009). Mathematical analysis of a two strain HIV/AIDS model with antiretroviral treatment. Acta Biotheor., 57(3), 361–381.
Bierman, W. F., van Agtmael, M. A., Nijhuis, M., Danner, S. A., & Boucher, C. A. (2009). HIV monotherapy with ritonavir-boosted protease inhibitors: a systematic review. AIDS, 23(3), 279–291.
Bilello, J. A., Bilello, P. A., Stellrecht, K., Leonard, J., Norbeck, D. W., Kempf, D. J., Robins, T., & Drusano, G. L. (1996). Human serum α 1 acid glycoprotein reduces uptake, intracellular concentration, and antiviral activity of a-80987, and inhibitor of the human immunodeficiency virus type-1 protease. Antimicrob. Agents Chemother., 40(6), 1491–1497.
Blower, S. M., & Dowlatabadi, H. (1994). Sensitivity and uncertainty analysis of complex models of disease transmission: an HIV model, as an example. Int. Stat. Rev., 2, 229–243.
Calza, L., & Manfredi, R. (2012). Protease inhibitor monotherapy as maintenance regimen in patients with HIV infection. Curr. HIV Res., 10(8), 661–672.
De Boer, R. J., Ribeiro, R. M., & Perelson, A. S. (2010). Current estimates for HIV-1 production imply rapid viral clearance in lymphoid tissues. PLoS Comput. Biol., 6(9), e1000906.
Diekmann, O., Heesterbeek, J. A. P., & Metz, J. A. (1990). On the definition and the computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations. J. Math. Biol., 28(4), 365.
Hale, J. K. (1969). Ordinary differential equations. New York: Wiley.
Heye, T. B., Tadesse, B. T., & Yalew, A. W. (2012). Predictors of treatment failure and time to detection and switching in HIV-infected Ethiopian children receiving first line anti-retroviral therapy. BMC Infect. Dis., 12(1).
Ho, D. D., Neuwann, A. U., Perelson, A. S., Chen, W., Leonard, J. M., & Markowitz, M. (1995). Rapid turnover of plasma visions and CD4 lymphocytes in HIV-1 infection. Nature, 373(6510), 123–126.
Huang, Y., Wu, H., & Acosta, E. P. (2010). Hierarchical Bayesian inference for HIV dynamic differential equation models incorporating multiple treatment factors. Biom. J., 52(4), 470–486.
Janeway, C., Travers, P., Walport, M., & Shlomchik, M. (2006). Immunobiology: the immune system in health and disease (6th ed., pp. 461–510). London: Garland Science, Taylor & Francis.
Kepler, T. B., & Perelson, A. S. (1998). Drug concentration heterogeneity facilitates the evolution of drug resistance. Proc. Natl. Acad. Sci. USA, 95, 11514–11519.
Kitayimbwa, J. M., Mugisha, J. Y., & Saenz, R. A. (2013). The role of backward mutations on the within-host dynamics of HIV-1. J. Math. Biol. 67(5), 1111–1139.
Krakovska, O., & Wahl, L. M. (2007). Optimal drug treatment regimens for HIV depend on adherence. J. Theor. Biol., 246(3), 499–509.
Lakshmikantham, V., Bainov, D. D., & Simeonov, P. S. (1989). Theory of impulsive differential equations. Singapore: World Scientific.
Lou, J., & Smith?, R. J. (2011). Modelling the effects of adherence to the HIV fusion inhibitor enfuvirtide. J. Theor. Biol., 268(1), 1–13.
Lou, J., Lou, Y., & Wu, J. (2012). Threshold virus dynamics with impulsive antiretroviral drug effects. J. Math. Biol. 65(4), 623–652.
Miron, R. M., & Smith?, R. J. (2010). Modelling imperfect adherence to HIV induction therapy. BMC Infect. Dis., 10(6).
Mohanty, U., & Dixit, N. M. (2008). Mechanism-based model of the pharmacokinetics of enfuvirtide, an HIV fusion inhibitor. J. Theor. Biol., 251(3), 541–551.
Okero, F. A., Aceng, E., Madraa, E., Namagala, E., & Serutoke, J. (2003). Scaling up antiretroviral therapy: experience in Uganda. Perspectives and practice in antiretroviral treatment. Case Study. World Health Organization and the Republic of Uganda.
Perelson, A. S., Kirschner, D. E., & De Boer, R. (1993). Dynamics of HIV infection of CD4+ T cells. Math. Biosci., 114, 81–125.
Perelson, A. S., Neumann, A. U., Markowitz, M., Leonard, J. M., & Ho, D. D. (1996). HIV-1 dynamics in vivo: virion clearance rate, infected cell lifespan, and viral generation time. Science, 271, 1582–1585.
Pillay, D., Bhaskaran, K., Jurriaans, S., Prins, M., Masquelier, B., Dabis, F., Gifford, R., Nielsen, C., Pedersen, C., Balotta, C., Rezza, G., Ortiz, M., de Mendoza, C., Kucherer, C., Poggensee, G., Gill, J., & Porter, K. (2006). The impact of transmitted drug resistance on the natural history of HIV infection and response to first-line therapy. AIDS, 20, 21–28.
Pillay, D., Goodall, R., Gilks, C. F., Yirrell, D., Gibb, D., Spyer, M., Kaleebu, P., Munderi, P., Kityo, C., McCormick, A., Nkalubo, J., Lyagoba, F., Chirara, M., & Hakim, J., DART (2010). Virological findings from the SARA trial: boosted PI monotherapy as maintenance second-line ART in Africa. J. Int. AIDS Soc., 13(Suppl 4), O20.
Rong, L., Feng, Z., & Perelson, A. S. (2007b). Emergence of HIV-1 drug resistance during antiretroviral treatment. Bull. Math. Biol., 69(6), 2027–2060.
Rong, L., Gilchrist, M. A., Feng, Z., & Perelson, A. S. (2007a). Modeling within-host HIV-1 dynamics and the evolution of drug resistance: trade-offs between viral enzyme function and drug susceptibility. J. Theor. Biol., 247(4), 804–818.
Rosenbloom, D. I., Hill, A. L., Rabi, S. A., Siliciano, R. F., & Nowak, M. A. (2012). Antiretroviral dynamics determines HIV evolution and predicts therapy outcome. Nat. Med. 18(9), 1378–1385.
Shi, V., Tridane, A., & Kuang, Y. (2008). A viral load-based cellular automata approach to modeling HIV dynamics and drug treatment. J. Theor. Biol., 253(1), 24–35.
Shiri, T., & Welte, A. (2008). Transient antiretroviral therapy selecting for common HIV-1 mutations substantially accelerates the appearance of rare mutations. Theor. Biol. Med. Model., 5, 25.
Smith?, R. J. (2006). Adherence to antiretroviral HIV drugs: how many doses can you miss before resistance emerges? Proc. R. Soc. Lond. Ser. B, Biol. Sci., 273(1586), 617–624.
Smith?, R. J., & Aggarwala, B. D. (2009). Can the viral reservoir of latently infected CD4+ T cells be eradicated with antiretroviral HIV drugs? J. Math. Biol., 59(5), 697–715.
Smith?, R. J., & Schwartz, E. J. (2008). Predicting the potential impact of a cytotoxic T-lymphocyte HIV vaccine: how often should you vaccinate and how strong should the vaccine be? Math. Biosci., 212, 180–187.
Smith?, R. J., & Wahl, L. M. (2004). Distinct effects of protease and reverse transcriptase inhibition in an immunological model of HIV-1 infection with impulsive drug effects. Bull. Math. Biol., 66, 1259–1283.
Smith?, R. J., & Wahl, L. M. (2005). Drug resistance in an immunological model of HIV-1 infection with impulsive drug effects. Bull. Math. Biol., 67, 783–813.
Stein, M. (1985). The use of LHS for variance reduction in simulations with many random parameters. IBM Report RC11166 (#50265) 52385, mathematics, 46 pp.
Truccolo, W. A., Rangarajan, G., Chen, Y., & Ding, M. (2003). Analysing stability of equilibrium points in neutral networks: a general approach. Neutral Netw., 16, 1453–1460.
van den Driessche, P., & Watmough, J. (2002). Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci., 180, 29.
von Wyl, V., Cambiano, V., Jordan, M. R., Bertagnolio, S., Miners, A., Pillay, D., Lundgren, J., & Phillips, A. N. (2012). Cost-effectiveness of tenofovir instead of zidovudine for use in first-line antiretroviral therapy in settings without virological monitoring. PLoS One, 7(8).
Wagner, B. G., & Blower, S. (2012). Universal access to HIV treatment versus universal ‘test and treat’: transmission, drug resistance & treatment costs. PLoS One, 7(9).
Wheeler, W. H., Ziebell, R. A., Zabina, H., Pieniazek, D., Prejean, J., Bodnar, U. R., Mahle, K. C., Heneine, W., Johnson, J. A., & Hall, H. I. (2010). Prevalence of transmitted drug resistance associated mutations and HIV-1 subtypes in new HIV-1 diagnoses, U.S.-2006. AIDS, 24(8), 1203–1212.
Wu, J., Yan, P., & Archibald, C. (2007). Modelling the evolution of drug resistance in the presence of antiviral drugs. BMC Public Health, 7, 300.
Acknowledgements
R.E.M. is funded by an Ontario Graduate Scholarship. R.J.S. is supported by an NSERC Discovery Grant, an Early Researcher Award and funding from MITACS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miron, R.E., Smith, R.J. Resistance to Protease Inhibitors in a Model of HIV-1 Infection with Impulsive Drug Effects. Bull Math Biol 76, 59–97 (2014). https://doi.org/10.1007/s11538-013-9903-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-013-9903-9