Abstract
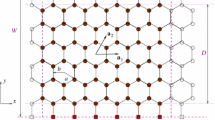
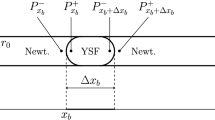
A numerical method is implemented for computing unsteady blood flow through a branching capillary network. The evolution of the discharge hematocrit along each capillary segment is computed by integrating in time a one-dimensional convection equation using a finite-difference method. The convection velocity is determined by the local and instantaneous effective capillary blood viscosity, while the tube to discharge hematocrit ratio is deduced from available correlations. Boundary conditions for the discharge hematocrit at divergent bifurcations arise from the partitioning law proposed by Klitzman and Johnson involving a dimensionless exponent, q≥1. When q=1, the cells are partitioned in proportion to the flow rate; as q tends to infinity, the cells are channeled into the branch with the highest flow rate. Simulations are performed for a tree-like, perfectly symmetric or randomly perturbed capillary network with m generations. When the tree involves more than a few generations, a supercritical Hopf bifurcation occurs at a critical value of q, yielding spontaneous self-sustained oscillations in the absence of external forcing. A phase diagram in the m–q plane is presented to establish conditions for unsteady flow, and the effect of various geometrical and physical parameters is examined. For a given network tree order, m, oscillations can be induced for a sufficiently high value of q by increasing the apparent intrinsic viscosity, decreasing the ratio of the vessel diameter from one generation to the next, or by decreasing the diameter of the terminal vessels. With other parameters fixed, oscillations are inhibited by increasing m. The results of the continuum model are in excellent agreement with the predictions of a discrete model where the motion of individual cells is followed from inlet to outlet.
Similar content being viewed by others
References
Bird, R. B., Stewart, W. E., & Lightfoot, E. N. (2001). Transport phenomena. New York: Wiley.
Carr, R. T., & Lacoin, M. (2000). Nonlinear dynamics of microvascular blood flow. Ann. Biomed. Eng., 28, 641–652.
Carr, R. T., Geddes, J. B., & Wu, F. (2005). Oscillations in a simple microvascular network. Ann. Biomed. Eng., 33, 764–771.
Fenton, B. M., Wilson, D. W., & Cokelet, G. R. (1985). Analysis of the effects of measured white blood cell entrance times on hemodynamics in a computer model of a microvascular bed. Pflügers Arch., 403, 396–400.
Geddes, J. B., Carr, R. T., Karst, N. J., & Wu, F. (2007). The onset of oscillations in microvascular blood flow. SIAM J. Appl. Dyn. Syst., 6, 694–727.
Iooss, G., & Joseph, D. D. (1990). Elementary stability and bifurcation theory (2nd ed.). New York: Springer.
Karshafian, R., Burns, P. N., & Henkelman, M. R. (2003). Transit time kinetics in ordered and disordered vascular trees. Phys. Med. Biol., 48, 3225–3237.
Kiani, M. F., Pries, A. R., Hsu, L. L., Sarelius, I. H., & Cokelet, G. R. (1994). Fluctuations in microvascular blood flow parameters caused by hemodynamic mechanisms. Am. J. Physiol., 266, H1822–H1828.
Klitzman, B., & Johnson, P. C. (1982). Capillary network geometry and red cell distribution in the hamster cremaster muscle. Am. J. Physiol., 242, H211–H219.
Lipowsky, H. H., & Zweifach, B. W. (1974). Network analysis of microcirculation of cat mesentery. Microvasc. Res., 7, 73–83.
Obrist, D., Weber, B., Buck, A., & Jenny, P. (2010). Red blood cell distribution in simplified capillary networks. Philos. Trans. R. Soc. A, 368, 2897–2918.
Price, R. J., & Skalak, T. C. (1995). A circumferential stress-growth rule predicts arcade arteriole formation in a network model. Microcirculation, 2, 41–51.
Pozrikidis, C. (2009). Numerical simulation of blood flow through microvascular capillary networks. Bull. Math. Biol., 71, 1520–1541.
Pries, A. R., Secomb, T. W., Gaehtgens, P., & Gross, J. F. (1990). Blood flow in microvascular networks. Experiments and simulation. Circ. Res., 67, 826–834.
Pries, A. R., Neuhaus, D., & Gaetgens, P. (1992). Blood viscosity in tube flow: dependence on diameter and hematocrit. Am. J. Physiol., 263, H1770–H1778.
Pries, A. R., Secomb, T. W., & Gaehtgens, P. (1996). Biophysical aspects of blood flow in the microvasculature. Cardiovasc. Res., 32, 654–667.
Secomb, T. W. (2003). Mechanics of red blood cells and blood flow in narrow tubes. In C. Pozrikidis (Ed.), Modeling and simulation of liquid capsules and biological cells. Boca Raton: Chapman & Hall/CRC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davis, J.M., Pozrikidis, C. Numerical Simulation of Unsteady Blood Flow through Capillary Networks. Bull Math Biol 73, 1857–1880 (2011). https://doi.org/10.1007/s11538-010-9595-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-010-9595-3