Abstract
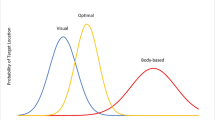
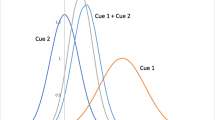
Uncertainty plays an important role in several navigational computations. Navigation typically depends on multiple sources of information, and different navigational systems may operate both in parallel and in combination. The optimal combination of information from different sources must take into account the uncertainty of that information. We distinguish between two types of spatial uncertainty, precision, and reliability. Precision is the inverse variance of the probability distribution that describes the information a cue contributes to an organism’s knowledge of its location. Reliability is the probability of the cue being correctly identified, or the probability of a cue being related to a target location. We argue that in most environments, precision and reliability are negatively correlated. In case of cue conflict, precision and reliability must be traded off against each other. We offer a quantitative description of optimal behaviour. Knowledge of uncertainty is also needed to optimally determine the point where a search should start when an organism has more precise spatial information in one of the spatial dimensions. We show that if there is any cost to travel, it is advantageous to head off to one side of the most likely target location and head toward the target. The magnitude of the optimal offset depends on both travel cost and search cost.
Similar content being viewed by others
References
Atkins, J. E., Fiser, J., & Jacobs, R. A. (2001). Experience-dependent visual cue integration based on consistencies between visual and haptic percepts. Vis. Res., 41, 449–461.
Biegler, R. (2000). Possible uses of path integration in animal navigation. Anim. Learn. Behav., 28(3), 257–277.
Biegler, R. (2006). Functional considerations in animal navigation: how do you use what you know? In M. F. Brown & R. G. Cook (Eds.), Animal spatial cognition: comparative, neural, and computational approaches. [On-line]. Available: www.pigeon.psy.tufts.edu/asc/biegler/.
Biegler, R., & Morris, R. G. M. (1996). Landmark stability: further studies pointing to a role in spatial learning. Q. J. Exp. Psychol., 49B(4), 307–345.
Bisetzky, A. R. (1957). Die Tänze der Bienen nach einem Fussweg zum Futterplatz. Z. Vgl. Physiol., 40, 264–288.
Chapuis, N., & Varlet, C. (1987). Short cuts by dogs in natural surroundings. Q. J. Exp. Psychol., 35B, 213–219.
Chapuis, N., Thinus-Blanc, C., & Poucet, B. (1983). Dissociation of mechanisms involved in dogs’ oriented displacements. Q. J. Exp. Psychol., 35B, 213–219.
Cheng, K. (1988). Some psychophysics of the pigeon’s use of landmarks. J. Comp. Physiol. A, 162, 815–826.
Cheng, K. (1990). More psychophysics of the pigeon’s use of landmarks. J. Comp. Physiol. A, 166, 857–863.
Cheng, K., Collett, T. S., Pickhard, A., & Wehner, R. (1987). The use of visual landmarks by honey bees: Bees weight landmarks according to their distance from the goal. J. Comp. Physiol. A, 161, 469–475.
Cheng, K., Shettleworth, S. J., et al. (2007). Bayesian integration of spatial information. Psychol. Bull., 133(4), 625–637.
Chittka, L., & Geiger, K. (1995a). The influences of landmarks on distance estimation of honey bees. Anum. Behav., 50, 23–31.
Chittka, L., & Geiger, K. (1995b). Honeybee long-distance orientation in a controlled environment. Ethology, 99, 117–126.
Dayan, P., & Yu, A. (2003). Uncertainty and learning. IETE J. Res., 49(2–3), 171–181.
Dall, S. R. X., Giraldeau, L. A., Olsson, O., McNamara, J. M., & Stephens, D. W. (2005). Information and its use by animals in evolutionary ecology. Trends Ecol. Evol., 20(4), 187–193.
Ellsberg, D. (1961). Risk, ambiguity, and the Savage axioms. Q. J. Econ., 75, 643–669.
Ernst, M. O. (2006). A Bayesian view on multimodal cue integration. In G. Knoblich, I. M. Thornton, M. Grosjean & M. Shiffrar (Eds.), Human body perception from the inside out (pp. 105–131). Oxford: Oxford University Press.
Ernst, M. O. (2007). Learning to integrate arbitrary signals from vision and touch. J. Vis., 7(5), 1–14.
Ernst, M. O., & Banks, M. (2002). Humans integrate visual and haptic information in a statistically optimal fashion. Nature, 415, 429–433.
Etienne, A. S., Teroni, E., Hurni, C., & Portennier, V. (1990). The effect of a single light cue on homing behaviour of the golden hamster. Anim. Behav., 39, 17–41.
Gatty, H. (1999). Finding your way without map or compass. Mineola: Dover.
Gothard, K. M., Skaggs, W. E., & McNaughton, B. L. (1996). The dynamics of mismatch correction in the hippocampal ensemble code for space: interaction between path integration and environmental cues. J. Neurosci., 16(24), 8027–8040.
Hampton, R. R. (2003). Metacognition as evidence for explicit representation in nonhumans. Behav. Brain Sci., 26, 346–347.
Hartmann, G., & Wehner, R. (1995). The ant’s path integration system: a neural architecture. Biol. Cybern., 73, 483–497.
Healy, S. D., Hurly, T. A. (1998). Rufous hummingbirds’ (Selasphorus rufus) memory for flowers: patterns or actual spatial locations? J. Exp. Psychol., Anim. Behav. Process., 24(4), 396–404.
Huettel, S. A., Stowe, C. J., Gordon, E. A., Warner, B. T., & Platt, M. L., (2006). Neural signatures of economic preferences for risk and ambiguity. Neuron, 49, 765–775.
Hsu, M., Bhatt, M., Adolphs, R., Tranel, D., & Camerer, C. F. (2005). Neural systems responding to degrees of uncertainty in human decision-making. Science, 310, 1680–1683.
Inglis, I. R. (2000). The central role of uncertainty reduction in determining behaviour. Behaviour, 137, 1567–1599.
Jacobs, R. A. (2002). What determines visual cue reliability? Trends Cogn. Sci., 6(8), 345–350.
Jazayeri, M., & Movshon, J. A. (2006). Optimal representation of sensory information by neural populations. Nat. Neurosci., 9(5), 690–696.
Jeffery, K. J. (1998). Learning of landmark stability and instability by hippocampal place cells. Neuropharmacology, 37(4–5), 677–687.
Kamil, A. C., & Jones, J. E. (1997). The seed-storing corvid Clark’s nutcracker learns geometric relationships among landmarks. Nature, 390, 276–279.
Knierim, J. J., Kudrimoti, H. S., & McNaughton, B. L. (1995). Place cells, head direction cells, and the learning of landmark stability. J. Neurosci., 15(3), 1648–1659.
Knill, D. C. (2007). Robust cue integration: a Bayesian model and evidence from cue-conflict studies with stereoscopic and figure cues to slant. J. Vis., 7(7).
Koerding, K. P., & Wolpert, D. M. (2004). Bayesian integration in sensorimotor learning. Nature, 427, 244–247.
Koops, M. A. (2004). Reliability and the value of information. Anim. Behaviour, 67, 103–111.
Ma, W. J., Beck, J. M., Latham, P. E., & Pouget, A. (2006). Bayesian inference with probabilistic population codes. Nat. Neurosci., 9(11), 1432–1438.
Mackintosh, J. H. (1973). Factors affecting the recognition of territory boundaries by mice (Mus musculus). Anim. Behav., 21, 464–470.
Maybeck, P. S. (1979). Stochastic models, estimation and control. New York: Academic Press.
Moser, E., & Moser, M. B. (2008). A metric for space. Hippocampus, 18(12), 1142–1156.
Müller, M., & Wehner, R. (1988). Path integration in desert ants, Cataglyphis fortis. Proc. Natl. Acad. Sci. USA, 85, 5287–5290.
Nardini, M., Jones, P., Bedford, R., & Braddick, O. (2008). Development of cue integration in human navigation. Curr. Biol., 18, 689–693.
O’Keefe, J., & Burgess, N. (1996). Geometric determinants of the place fields of hippocampal neurons. Nature, 381, 425–428.
Pfuhl, G., Tjelmeland, H., & Biegler, R. (2009). Optimal cache search depends on precision of spatial memory and pilfering, but what if that knowledge is not perfect? Anim. Behav., 78, 819–828.
Roach, N. W., Heron, J., & et al. (2006). Resolving multisensory conflict: a strategy for balancing the costs and benefits of audio-visual integration. Proc. R. Soc. B, Biol. Sci., 273(1598), 2159–2168.
Roberts, A. D. L., & Pearce, J. M. (1998). Control of spatial behavior by an unstable landmark. J. Exp. Psychol. Anim. Behav. Process., 24(2), 172–184.
Rotenberg, A., & Muller, R. U. (1997). Variable place-cell coupling to a continuously viewed stimulus: evidence that the hippocampus acts as a perceptual system. Philos. Trans. R. Soc. Lond. B, 352, 1505–1513.
Sato, Y., Toyoizumi, T., & Aihara, K. (2007). Bayesian inference explains perception of unity and ventriloquism aftereffect: identification of common sources of audiovisual stimuli. Neur. Comput., 19, 3335–3355.
Séguinot, V., Maurer, R., & Etienne, A. S. (1993). Dead reckoning in a small mammal: the evaluation of distance. J. Comp. Physiol. A, 173, 103–113.
Seyfarth, E. A., Hergenröder, R., Ebbes, H., & Barth, F. G. (1982). Idiothetic orientation of a wandering spider: compensation of detours and estimates of global distance. Behav. Ecol. Sociobiol., 11, 139–148.
Shettleworth, S. J., & Sutton, J. E. (2003). Animal metacognition? It’s all in the menthods. Behav. Brain Sci., 26, 353–354.
Smith, J. D., Shields, W. E., & Washburn, D. A. (2003). The comparative psychology of uncertainty monitoring and metacognition. Behav. Brain Sci., 26, 317–373.
Stephens, D. W. (1989). Variance and the value of information. Am. Nat., 134(1), 128–140.
Wehner, R., & Srinivasan, M. V. (1981). Searching behaviour of desert ants, genus Cataglyphis (Formicidae, Hymenoptera). J. Comp. Physiol., 142, 315–338.
Wolf, H., & Wehner, R. (2000). Pinpointing food sources: olfactory and anemotactic orientation in desert ants, cataglyphis bicolour. J. Exp. Biol., 203, 857–868.
Wolf, H., & Wehner, R. (2005). Desert ants compensate for navigation uncertainty. J. Exp. Biol., 208, 4223–4230.
Yu, A. J., & Dayan, P. (2003). Expected and unexpected uncertainty. Ach and NE in the neocortex. In L. K. Saul, Y. Weiss, & L. Bottou (Eds.), Advances in neural information processing systems 17 (pp. 1577–1584). Cambridge: MIT Press.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pfuhl, G., Tjelmeland, H. & Biegler, R. Precision and Reliability in Animal Navigation. Bull Math Biol 73, 951–977 (2011). https://doi.org/10.1007/s11538-010-9547-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-010-9547-y