Abstract
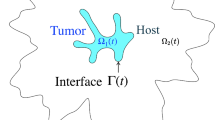
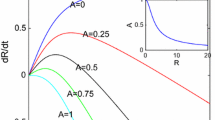
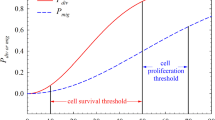
We study the interface morphology of a 2D simulation of an avascular tumor composed of identical cells growing in an homogeneous healthy tissue matrix (TM), in order to understand the origin of the morphological changes often observed during real tumor growth. We use the Glazier–Graner–Hogeweg model, which treats tumor cells as extended, deformable objects, to study the effects of two parameters: a dimensionless diffusion-limitation parameter defined as the ratio of the tumor consumption rate to the substrate transport rate, and the tumor-TM surface tension. We model TM as a nondiffusing field, neglecting the TM pressure and haptotactic repulsion acting on a real growing tumor; thus, our model is appropriate for studying tumors with highly motile cells, e.g., gliomas. We show that the diffusion-limitation parameter determines whether the growing tumor develops a smooth (noninvasive) or fingered (invasive) interface, and that the sensitivity of tumor morphology to tumor-TM surface tension increases with the size of the dimensionless diffusion-limitation parameter. For large diffusion-limitation parameters, we find a transition (missed in previous work) between dendritic structures, produced when tumor-TM surface tension is high, and seaweed-like structures, produced when tumor-TM surface tension is low. This observation leads to a direct analogy between the mathematics and dynamics of tumors and those observed in nonbiological directional solidification. Our results are also consistent with the biological observation that hypoxia promotes invasive growth of tumor cells by inducing higher levels of receptors for scatter factors that weaken cell-cell adhesion and increase cell motility. These findings suggest that tumor morphology may have value in predicting the efficiency of antiangiogenic therapy in individual patients.
Similar content being viewed by others
References
Anderson, A.R.A., 2005. A hybrid mathematical model of solid tumour invasion: the importance of cell adhesion. Math. Med. Biol. 22, 163.
Anderson, A.R.A., Chaplain, M.A.J., 1998. Continuous and discrete mathematical models of tumourinduced angiogenesis. Bull. Math. Biol. 60, 857.
Anderson, A.R.A., Chaplain, M.A.J., Newman, E.L., Steele, R.J.C., Thompson, A.M., 2000. Mathematical modelling of tumour invasion and metastasis. J. Theor. Med. 2, 129.
Anderson, A.R.A., Weaver, A.M., Cummings, P.T., Quaranta, V., 2006. Tumor morphology and phenotypic evolution driven by selective pressure from the microenvironment. Cell 127, 905.
Anderson, A.R.A., Rejniak, K.A., Gerlee, P., Quaranta, V., 2009. Microenvironment driven invasion: a multiscale multimodel investigation. J. Math. Biol. 58, 579.
Balter, A., Merks, R.M.H., Popławski, N.J., Swat, M., Glazier, J.A., 2007. The Glazier–Graner–Hogeweg model: extensions, future directions, and opportunities for further study. In: Anderson, A.R.A., Chaplain, M.A.J., Rejniak, K.A. (Eds.), Single-Cell-Based Models in Biology and Medicine, p. 151. Birkhäuser, Basel.
Bechhoefer, J., Liebchaber, A., 1987. Testing shape selection in directional solidification. Phys. Rev. B 35, 1393.
Beysens, D.A., Forgacs, G., Glazier, J.A., 2000. Embryonic tissues are viscoelastic materials. Can. J. Phys. 78, 243.
Blagosklonny, M.V., 2001. Hypoxia-inducible factor: Achilles’ heel of antiangiogenic cancer therapy. Int. J. Oncol. 19, 257.
Bray, D., 1992. Cell Movements. Garland, New York.
Brener, E., Müller-Krumbhaar, H., Temkin, D., 1992. Kinetic phase diagram and scaling relations for stationary diffusional growth. Europhys. Lett. 17, 535.
Brú, A., Pastor, J.M., Fernaud, I., Melle, S., Brú, I., 1998. Super-rough dynamics on tumour growth. Phys. Rev. Lett. 81, 4008.
Brú, A., Albertos, S., Subiza, J.L., García-Asenjo, J.L., Brú, I., 2003. The universal dynamics of tumor growth. Biophys. J. 85, 2948.
Burgess, P.K., Kulesa, P.M., Murray, J.D., Alvord, E.C., 1997. Growth patterns of microscopic brain tumors. J. Neuropathol. Exp. Neurol. 56, 704.
Burridge, K., Chrzanowska-Wodnicka, M., 1996. Focal adhesions, contractability, and signalling. Annu. Rev. Cell Dev. Biol. 12, 463.
Byrne, H.M., Chaplain, M.A.J., Pettet, G.J., McElwain, D.L.S., 1999. A mathematical model of trophoblast invasion. J. Theor. Med. 1, 275.
Calabresi, P., Schein, P.S., 1993. Medical Oncology, 2nd edn. McGraw-Hill, New York.
Carter, S.B., 1965. Principles of cell motility: the direction of cell movement and cancer invasion. Nature 208, 1183.
Casciari, J.J., Sotirchos, S.V., Sutherland, R.M., 1988. Glucose diffusivity in multicellular tumor spheroids. Cancer. Res. 48, 3905.
Casciari, J.J., Sotirchos, S.V., Sutherland, R.M., 1992. Variation in tumour cell growth rates and metabolism with oxygen-concentration, glucose-concentration and extracellular pH. J. Cell. Physiol. 151, 386.
Chambers, A.F., Matrisian, L.M., 1997. Changing views of the role of matrix metalloproteinases in metastasis. J. Natl. Cancer Inst. 89, 1260.
Chaplain, M.A.J., 1996. Avascular growth, angiogenesis and vascular growth in solid tumours: the mathematical modelling of the stages of tumour development. Math. Comput. Model. 23, 47.
Chaplain, M.A.J., Sleeman, B.D., 1993. Modelling the growth of solid tumours and incorporating a method for their classification using nonlinear elasticity theory. J. Math. Biol. 31, 431.
Chapman, G., Pardy, R.L., 1972. The movement of glucose and glycine through the tissues of Corymorpha palma torrey (coelenterata, hydrozoa). J. Exp. Biol. 56, 639.
Chaturvedi, R., Izaguirre, J.A., Huang, C., Cickovski, T., Virtue, P., Thomas, G.L., Forgacs, G., Alber, M.S., Newman, S.A., Glazier, J.A., 2003. Multi-model simulations of chicken limb morphogenesis. Lect. Notes Comput. Sci. 2659, 39.
Chaturvedi, R., Huang, C., Izaguirre, J.A., Newman, S.A., Glazier, J.A., Alber, M., 2004. A hybrid discrete-continuum model for 3-D skeletogenesis of the vertebrate limb. Lect. Notes Comput. Sci. 3305, 543.
Chaturvedi, R., Huang, C., Kazmierczak, B., Schneider, T., Izaguirre, J.A., Glimm, T., Hentschel, H.G.E., Glazier, J.A., Newman, S.A., Alber, M.S., 2005. On multiscale approaches to three-dimensional modeling of morphogenesis. J. R. Soc. Interf. 2, 237.
Christofori, G., 2006. New signals from the invasive front. Nature 441, 444.
Cickovski, T.M., Huang, C., Chaturvedi, R., Glimm, T., Hentschel, H.G.E., Alber, M.S., Glazier, J.A., Newman, S.A., Izaguirre, J.A., 2005. A framework for three-dimensional simulation of morphogenesis. IEEE/ACM Trans. Comput. Biol. Bioinf. 2, 1.
Clark, E.A., Brugge, J.S., 1995. Integrins and signal transduction pathways: the road taken. Science 268, 233.
Condeelis, J., Singer, R.H., Segall, J.E., 2005. The great escape: When cancer cells hijack the genes for chemotaxis and motility. Annu. Rev. Cell Dev. Biol. 21, 695.
Cristini, V., Lowengrub, J., Nie, Q., 2003. Nonlinear simulation of tumor growth. J. Math. Biol. 46, 191.
Cristini, V., Frieboes, H.B., Gatenby, R., Caserta, S., Ferrari, M., Sinek, J., 2005. Morphologic instability and cancer invasion. Clin. Cancer Res. 11, 6772.
Davis, S.H., 2001. Theory of Solidification. Cambridge University Press, Cambridge.
Debruyne, P.R., Bruyneel, E.A. , 2002. Bile acids stimulate invasion and haptotaxis in human corectal cancer cells through activation of multiple oncogenic signalling pathways. Oncogene 21, 6740.
Dockery, J., Klapper, I., 2002. Finger formation in biofilm layers. SIAM J. Appl. Math. 62, 853.
Dormann, S., Deutsch, A., 2002. Modeling of self-organized avascular tumor growth with a hybrid cellular automaton. In Silico Biol. 2, 0035.
Drasdo, D., Höhme, S., 2003. Individual-based approaches to birth and death in avascular tumors. Math. Comput. Model. 37, 1163.
Dubuc, B., Quiniou, J.F., Roques-Carmes, C., Tricot, C., Zucker, S.W., 1989. Evaluating the fractal dimension of profiles. Phys. Rev. A 39, 1500.
Düchting, W., 1990. Tumor growth simulation. Comput. Graph. 14, 505.
Düchting, W., Ulmer, W., Ginsberg, T., 1996. Cancer: a challenge for control theory and computer modelling. Eur. J. Cancer 32A, 1283.
Folkman, J., 1995. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature Med. 1, 21.
Folkman, J., Hochberg, M., 1973. Self-regulation of growth in three dimensions. J. Exp. Med. 138, 745.
Forgacs, G., Foty, R.A., Shafrir, Y., Steinberg, M.S., 1998. Viscoelastic properties of living embryonic tissues: a quantitative study. Biophys. J. 74, 2227.
Foty, R.A., Forgacs, G., Pfleger, C.M., Steinberg, M.S., 1994. Liquid properties of embryonic tissues: measurement of interfacial tensions. Phys. Rev. Lett. 72, 2298.
Foty, R.A., Pfleger, C.M., Forgacs, G., Steinberg, M.S., 1996. Surface tensions of embryonic tissues predict their mutual envelopment behavior. Development 122, 1611.
Freyer, J.P., Tustanoff, E., Franko, A.J., Sutherland, R.M., 1984. In situ consumption rates of cells in v-79 multicellular spheroids during growth. J. Cell. Physiol. 118, 53.
Frieboes, H.B., Zheng, X., Sun, C., Tromberg, B., Gatenby, R., Cristini, V., 2006. An integrated computational/experimental model of tumor invasion. Cancer Res. 66, 1597.
Frieboes, H.B., Lowengrub, J.S., Wise, S., Zheng, X., Macklin, P., Elaine, L.B.D., Cristini, V., 2007. Computer simulation of glioma growth and morphology. Neuroimage 37, S59.
Friedel, P., Hegerfeldt, Y., Tusch, M., 2004. Collective cell migration in morphogenesis and cancer. Int. J. Dev. Biol. 48, 441.
Gatenby, R.A., Gawlinski, E.T., 1996. A reaction-diffusion model of cancer invasion. Cancer Res. 56, 5745.
Gerlee, P., Anderson, A.R.A., 2007a. An evolutionary hybrid cellular automaton model of solid tumour growth. J. Theor. Biol. 246, 583.
Gerlee, P., Anderson, A.R.A., 2007b. Stability analysis of a hybrid cellular automaton model of cell colony growth. Phys. Rev. E 75, 051911.
Gerlee, P., Anderson, A.R.A., 2007c. Stability analysis of a hybrid cellular automaton model of cell colony growth. Phys. Rev. E 75, 051911.
Gherardi, E., Gray, J., Stoker, M., Perryman, M., Furlong, R., 1989. Purification of scatter factor, a fibroblast-derived basic protein that modulates epithelial interactions and movement. Proc. Natl. Acad. Sci. USA 86, 5844.
Glazier, J.A., Graner, F., 1993. Simulation of the differential adhesion driven rearrangement of biological cells. Phys. Rev. E 47, 2128.
Glazier, J.A., Balter, A., Popławski, N.J., 2007. Magnetization to morphogenesis: a brief history of the Glazier-Graner-Hogeweg model. In: Anderson, A.R.A., Chaplain, M.A.J., Rejniak, K.A. (Eds.), Single-Cell-Based Models in Biology and Medicine, p. 79. Birkhäuser, Basel.
Glicksman, M.E., Lowengrub, J.S., Li, S.W., Li, X.R., 2007. A deterministic mechanism for dendritic solidification kinetics. JOM 59, 27.
Graner, F., Glazier, J.A., 1992. Simulation of biological cell sorting using a two-dimensional extended Potts model. Phys. Rev. Lett. 69, 2013.
Guiot, C., Delsanto, P.P., Deisboeck, T.S., 2007. Morphological instability and cancer invasion: a ‘splashing water drop’ analogy. Theor. Biol. Med. Model. 4, 4.
Hartmann, D., Miura, T., 2006. Modelling in vitro lung branching morphogenesis during development. J. Theor. Biol. 242, 862.
Holm, E.A., Glazier, J.A., Srolovitz, D.J., Grest, G.S., 1991. Effects of lattice anisotropy and temperature on domain growth in the two-dimensional Potts model. Phys. Rev. A 43, 2662.
Hotary, K., Allen, E.D., Brooks, P.C., Datta, N.S., Long, M.W., Weiss, S.J., 2003. Membrane type 1 matrix metalloproteinase usurps tumour growth control imposed by the three-dimensional extracellular matrix. Cell 114, 33.
Huang, S., Ingber, D.E., 1999. The structural and mechanical complexity of cell-growth control. Nat. Cell Biol. 1, E131.
Huber, M.A., Kraut, N., Beug, H., 2005. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 17, 548.
Hynes, R.O., 1992. Integrins: versatility, modulation, and signalling in cell adhesion. Cell 69, 11.
Izaguirre, J.A., Chaturvedi, R., Huang, C., Cickovski, T., Coffland, J., Thomas, G.L., Forgacs, G., Alber, M.S., Hentschel, H.G.E., Newman, S.A., Glazier, J.A., 2004. CompuCell, a multi-model framework for simulation of morphogenesis. Bioinformatics 20, 1129.
Jain, R.K., 1987. Growth patterns of microscopic brain tumors. Cancer Res. 47, 3039.
Jiang, Y., Pjesivac-Grbovic, J., Cantrell, C., Freyer, J.P., 2005. A multiscale model for avascular tumor growth. Biophys. J. 89, 3884.
Johansson, N., Ahonen, M., Kahari, V.-M., 2000. Matrix metalloproteinases in tumour invasion. Cell. Mol. Life Sci. 57, 5.
Kansal, A.R., Torquato, S., Harsh, G.R., Chiocca, E.A., Deisboeck, T.S., 2000. Simulated brain tumor growth using a three-dimensional cellular automaton. J. Theor. Biol. 203, 367.
Khain, E., Sander, L.M., 2006. Dynamics and pattern formation in invasive tumor growth. Phys. Rev. Lett. 96, 188103.
Kimmel, M., Axelrod, D.E., 1991. Unequal cell division, growth regulation and colony size of mammalian cells: a mathematical model and analysis of experimental data. J. Theor. Biol. 153, 157.
Klominek, J., Robert, K.H., Sundqvist, K.-G., 1993. Chemotaxis and haptotaxis of human malignant mesothelioma cells: effects of fibronectin, laminin, type iv collagen, and an autocrine motility factor-like substance. Cancer Res. 53, 4376.
Kobayashi, R., 1993. Modeling and numerical simulations of dendritic crystal growth. Physica D 63, 410.
Koochekpour, S., Pilkington, G.J., Merzak, A., 1995. Hyaluronic acid/CD44H interaction induces cell detachment and stimulates migration and invasion of human glioma cells in vitro. Int. J. Cancer 63, 450.
Kumar, N., Zaman, M.H., Kim, H.-D., Lauffenburger, D.A., 2006. A high-throughput migration assay reveals HER2-mediated cell migration arising from increased directional persistence. Biophys. J. 91, L32.
Lacovara, J., Cramer, E.B., Quigley, J.P., 1984. Fibronectin enhancement of directed migration of B16 melanoma cells. Cancer Res. 44, 1657.
Landau, L.D., Lifshitz, E.M., 1986. Theory of Elasticity. Pergamon, Elmsford.
Langer, J.S., 1980. Instabilities and pattern formation in crystal growth. Rev. Mod. Phys. 52, 1.
Langer, J.S., 1987. Lectures in the theory of pattern formation. In: Chance and Matter, p. 629. Elsevier, Amsterdam.
Lawrence, J.A., Steeg, P.S., 1996. Mechanisms of tumour invasion and metastasis. World J. Urol. 14, 124.
Lee, J.M., Dedhar, S., Kalluri, R., Thompson, E.W., 2006. The epithelial-mesenchymal transition: New insights in signaling, development, and disease. J. Cell Biol. 172, 973.
Li, X.R., Cristini, V., Nie, Q., Lowengrub, J.S., 2007. Nonlinear three-dimensional simulation of solid tumor growth. Discrete Contin. Dyn. Syst. Ser. B 7, 581.
Liotta, L.A., Kohn, E.C., 2001. The microenvironment of the tumour-host interface. Nature 411, 375.
Liotta, L.A., Rao, C.N., Barsky, S.H., 1983. Tumour invasion and the extracellular matrix. Lab. Invest. 49, 636.
Ludwig, A., 1999. Dendritic and cellular doublets: Morphologies of thin solid films growing along a substrate during the initial state of solidification of bulk melts. Phys. Rev. E 59, 1893.
Macklin, P., Lowengrub, J., 2007. Nonlinear simulation of the effect of microenvironment on tumor growth. J. Theor. Biol. 245, 677.
Mariani, L., Beaudry, C., McDonough, W.S., Hoelzinger, D.B., Demuth, T., Ross, K.R., Berens, T., Coons, S.W., Watts, G., Trent, J.M., Wei, J.S., Giese, A., Berens, M.E., 2001. Glioma cell motility is associated with reduced transcription of proapoptotic and proliferation genes: a cDNA microarray analysis. J. Neuro-Oncol. 53, 161.
Marusic, M., Bajzer, Z., Freyer, J.P., Vuk-Pavlovic, S., 1994. Analysis of growth of multicellular tumour spheroids by mathematical models. Cell Prolif. 27, 73.
Matrisian, L.M., 1992. The matrix-degrading metalloproteinases. Bioessays 14, 455.
McCarthy, J.B., Furcht, L.T., 1984. Laminin and fibronectin promote the directed migration of B16 melanoma cells in vitro. J. Cell Biol. 98, 1474.
Melicow, M.M., 1982. The three-steps to cancer: a new concept of carcinogenesis. J. Theor. Biol. 94, 471.
Merks, R.M.H., Glazier, J.A., 2005. A cell-centered approach to developmental biology. Physica A 352, 113.
Metropolis, N., Rosenbluth, A.W., Rosenbluth, M.N., Teller, A.H., Teller, E., 1953. Equation of state calculations by fast computing machines. J. Chem. Phys. 21, 1087.
Mignatti, P., Rifkin, D.B., 1993. Biology and biochemistry of proteinases in tumor invasion. Physiol. Rev. 73, 161.
Miura, T., Shiota, K., 2002. Depletion of FGF acts as a lateral inhibitory factor in lung branching morphogenesis in vitro. Mech. Dev. 116, 29.
Mombach, J.C., Glazier, J.A., Raphael, R.C., Zajac, M., 1995. Quantitative comparison between differential adhesion models and cell sorting in the presence and absence of fluctuations. Phys. Rev. Lett. 75, 2244.
Moore, M.G., Juel, A., Burgess, J.M., McCormick, W.D., Swinney, H.L., 2002. Fluctuations in viscous fingering. Phys. Rev. E 65, 030601(R).
Müller-Krumbhaar, H., Kurz, W., Brener, E., 1991. Solidification aa. In: Phase Tranformations in Materials. VCH, Weinheim.
Nakamura, T., Teramoto, H., Ichihara, A., 1986. Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proc. Natl. Acad. Sci. USA 83, 6489.
Nakamura, T., Nishizawa, T., Hagiya, M., Seki, T., Shimonishi, M., Sugimura, A., Tashiro, K., Shimizu, S., 1989. Molecular cloning and expression of human hepatocyte growth factor. Nature 342, 440.
Orme, M.E., Chaplain, M.A.J., 1996. A mathematical model of vascular tumour growth and invasion. Math. Comput. Model. 23, 43.
Pennacchietti, S., Michieli, P., Galluzzo, M., Mazzone, M., Giordano, S., Comoglio, P.M., 2003. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 3, 347.
Perumpanani, A.J., Sherratt, J.A., Norbury, J., Byrne, H.M., 1996. Biological inferences from a mathematical model of malignant invasion. Invasion Metastasis 16, 209.
Picioreanu, C., van Loosdrecht, M.C.M., Heijnen, J.J., 1998a. Mathematical modeling of biofilm structure with a hybrid differential-discrete cellular automaton approach. Biotechnol. Bioeng. 58, 101.
Picioreanu, C., van Loosdrecht, M.C.M., Heijnen, J.J., 1998b. A new combined differential-discrete cellular automaton approach for biofilm modeling: application for growth in gel beads. Biotechnol. Bioeng. 57, 718.
Pocheau, A., Georgelin, M., 2006. Shape of growth cells in directional solidification. Phys. Rev. E 73, 011604.
Popławski, N.J., Swat, M., Gens, J.S., Glazier, J.A., 2007. Adhesion between cells, diffusion of growth factors, and elasticity of the AER produce the paddle shape of the chick limb. Physica A 373, 521.
Popławski, N.J., Shirinifard, A., Swat, M., Glazier, J.A., 2008. Simulation of single-species bacterial-biofilm growth using the Glazier-Graner-Hogeweg model and the CompuCell3D modeling environment. Math. Biosci. Eng. 5, 355.
Qi, A., Zheng, X., Du, C., An, B., 1993. A cellular automaton model of cancerous growth. J. Theor. Biol. 161, 1.
Quigley, J.P., Lacovara, J., Cramer, E.B., 1983. The directed migration of B-16 melanoma-cells in response to a haptotactic chemotactic gradient of fibronectin. J. Cell Biol. 97, A450.
Radotra, B., McCormick, D., Cockard, A., 1994. CD44 plays a role in adhesive interactions between glioma cells and extracellular matrix components. Neuropathol. Appl. Neurobiol. 20, 399.
Rejniak, K.A., 2005. A single-cell approach in modeling the dynamics of tumor microregions. Math. Biosci. Eng. 2, 643.
Retsky, M.W., Swartzendruber, D.E., Wardwell, R.H., Bame, P.D., 1990. Is gompertzian or exponential kinetics a valid description of individual human cancer growth? Med. Hypotheses 33, 95.
Rubin, J.S., Bottaro, D.P., Aaronson, S.A., 1993. Hepatocyte growth factor/scatter factor and its receptor, the c-met proto-oncogene product. Biochim. Biophys. Acta 1155, 357.
Sahlgren, C., Gustafsson, M.V., Jin, S., Poellinger, L., Lendahl, U., 2008. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc. Natl. Acad. Sci. USA 105, 6392.
Saito, Y., Misbah, C., Müller-Krumbhaar, H., 1989. Directional solidification: Transition from cells to dendrites. Phys. Rev. Lett. 63, 2377.
Sander, L.M., Deisboeck, T.S., 2002. Growth patterns of microscopic brain tumors. Phys. Rev. E 66, 051901.
Sherratt, J.A., Nowak, M.A., 1992. Oncogenes, anti-oncogenes and the immune response to cancer: a mathematical model. Proc. R. Soc. Lond. B 248, 261.
Sherwood, L., 2001. Human Physiology: From Cells to Systems, 4th edn. Brooks/Cole, Belmont.
Smolle, J., Stettner, H., 1993. Computer simulation of tumour cell invasion by a stochastic growth model. J. Theor. Biol. 160, 63.
Stalder, I., Bilgram, J.H., 2001. Morphology of structures in diffusional growth in three dimensions. Europhys. Lett. 56, 829.
Steinberg, M.S., 1963. Reconstruction of tissues by dissociated cells. Some morphogenetic movements and the sorting out of embryonic cells may have a common explanation. Science 141, 401.
Stetler-Stevenson, W.G., Aznavoorian, S., Liotta, L.A., 1993. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu. Rev. Cell Biol. 9, 541.
Stetler-Stevenson, W.G., Hewitt, R., Corcoran, M., 1996. Matrix metallo-proteinases and tumour invasion: from correlation to causality to the clinic. Cancer Biol. 7, 147.
Stoker, N., Gherardi, E., Perryman, M., Grey, J., 1987. Scatter factor is a fibroblast-derived modulator of epithelial cell motility. Nature 327, 239.
Stott, E.L., Britton, N.F., Glazier, J.A., Zajac, M., 1999. Stochastic simulation of benign avascular tumor growth using the Potts model. Math. Comput. Mod. 30, 183.
Swanson, K.R., Bridge, C., Murray, J.D., Jr, E.C.A., 2003. Virtual and real brain tumors: using mathematical modeling to quantify glioma growth and invasion. J. Neurol. Sci. 216, 1.
Terranova, V.P., Diflorio, R., Lyall, R.M., Hic, S., Friesel, R., Maciag, T., 1985. Human endothelial cells are chemotactic to endothelial cell growth factor and heparin. J. Cell Biol. 101, 2330.
Thorgeirsson, U.P., Lindsay, C.K., Cottam, D.W., Gomez, D.E., 1994. Tumor invasion, proteolysis, and angiogenesis. J. Neurooncol. 18, 89.
Tracqui, P., 1995. From passive diffusion to active cellular migration in mathematical models of tumour invasion. Acta Biotheor. 43, 443.
Trédan, O., Galmarini, C.M., Patel, K., Tannock, I.F., 2007. Drug resistance and the solid tumor microenvironment. J. Natl. Cancer Inst. 99, 1441.
Trusolino, L., Comoglio, P.M., 2002. Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nature Rev. Cancer 4, 289.
Turner, S., Sherratt, J.A., 2002. Intercellular adhesion and cancer invasion: a discrete simulation using the extended Potts model. J. Theor. Biol. 216, 85.
Turner, S., Sherratt, J.A., Painter, K.J., Savill, N.J., 2004a. From a discrete to a continuous model of biological cell movement. Phys. Rev. E 69, 021910.
Turner, S., Sherratt, J.A., Cameron, D., 2004b. Tamoxifen treatment failure in cancer and the nonlinear dynamics of TGFβ. J. Theor. Biol. 229, 101.
Ward, J.P., King, J.R., 1999. Mathematical modelling of avascular-tumour growth II: modelling growth saturation. IMA J. Math. Appl. Med. Biol. 16, 171.
Weinberg, R.A., 2006. The Biology of Cancer. Garland, New York.
Wheldon, T.E., 1986. Mathematical models in experimental and clinical oncology. In: Ingram, D., Bloch, R.F. (Eds.), Mathematical Methods in Medicine, p. 1. Wiley, New York.
Witten, T.A., Sander, L.M., 1983. Diffusion-limited aggregation. Phys. Rev. B 27, 5686.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Popławski, N.J., Agero, U., Gens, J.S. et al. Front Instabilities and Invasiveness of Simulated Avascular Tumors. Bull. Math. Biol. 71, 1189–1227 (2009). https://doi.org/10.1007/s11538-009-9399-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-009-9399-5