Abstract
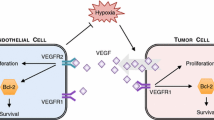
Recent experiments show that vascular endothelial growth factor (VEGF) is the crucial mediator of downstream events that ultimately lead to enhanced endothelial cell survival and increased vascular density within many tumors. The newly discovered pathway involves up-regulation of the anti-apoptotic protein Bcl-2, which in turn leads to increased production of interleukin-8 (CXCL8). The VEGF–Bcl-2–CXCL8 pathway suggests new targets for the development of anti-angiogenic strategies including short interfering RNA (siRNA) that silence the CXCL8 gene and small molecule inhibitors of Bcl-2. In this paper, we present and validate a mathematical model designed to predict the effect of the therapeutic blockage of VEGF, CXCL8, and Bcl-2 at different stages of tumor progression. In agreement with experimental observations, the model predicts that curtailing the production of CXCL8 early in development can result in a delay in tumor growth and vascular development; however, it has little effect when applied at late stages of tumor progression. Numerical simulations also show that blocking Bcl-2 up-regulation, either at early stages or after the tumor has fully developed, ensures that both microvascular and tumor cell density stabilize at low values representing growth control. These results provide insight into those aspects of the VEGF–Bcl-2–CXCL8 pathway, which independently and in combination, are crucial mediators of tumor growth and vascular development. Continued quantitative modeling in this direction may have profound implications for the development of novel therapies directed against specific proteins and chemokines to alter tumor progression.
Similar content being viewed by others
References
Anderson, A.R., Chaplain, M.A., 1998. Continuous and discrete mathematical models of tumor-induced angiogenesis. Bull. Math. Biol. 60(5), 857–899.
Ausprunk, D.H., Folkman, J., 1977. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc. Res. 14(1), 53–65.
Bach, F., Uddin, F.J., Burke, D., 2007. Angiopoietins in malignancy. Eur. J. Surg. Oncol. 33(1), 7–15.
Baxter, L.T., Jain, R.K., 1991. Transport of fluid and macromolecules in tumors. III. Role of binding and metabolism. Microvasc. Res. 41(1), 5–23.
Bernatchez, P.N., Soker, S., Sirois, M.G., 1999. Vascular endothelial growth factor effect on endothelial cell proliferation, migration, and platelet-activating factor synthesis is Flk-1-dependant. J. Biol. Chem. 274(43), 31047–31054.
Cao, Y., 2004. Antiangiogenic cancer therapy. Semin. Cancer Biol. 14(2), 139–145.
Chaplain, M.A., Anderson, A.R., 1996. Mathematical modelling, simulation and prediction of tumour-induced angiogenesis. Invasion Metastasis 16(4-5), 222–234.
Daugulis, P., Arakelyan, L., Ginosar, Y., Agur, Z., 2004. Hopf point analysis for angiogenesis models. Discret. Contin. Dyn. Syst. Ser. B 4(1), 29–38.
Dong, Z., Song, W., Sun, Q., Zeitlin, B.D., Karl, E., Spencer, D.M., Jain, H.V., Jackson, T., Núñez, G., Nör, J.E., 2007. Endothelial cell apoptosis and microvessel disruption. Exp. Cell Res., accepted.
Dvorak, H.F., Brown, L.F., Detmar, M., Dvorak, A.M., 1995. Vascular permeability factor/vascular endothelial growth factor, vascular hyperpermeability, and angiogenesis. Am. J. Pathol. 146(5), 1029–1039.
Ferrara, N., 1999. Molecular and biological properties of vascular endothelial growth factor. J. Mol. Med. 77(7), 527–543.
Ferrara, N., 2002. VEGF and the quest for tumour angiogenesis factors. Nat. Rev. Cancer 2, 795–803.
Ferrara, N., Gerber, H.P., LeCouter, J., 2003. The biology of VEGF and its receptors. Nat. Med. 9(6), 669–676.
Folkman, J., 1971. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 285(21), 1182–1186.
Gammack, D., Byrne, H.M., 2001. Estimating the selective advantage of mutant p53 tumour cells to repeated rounds of hypoxia. Bull. Math. Biol. 63(1), 135–166.
Garber, K., 2002. Angiogenesis inhibitors suffer new setback. Nat. Biotechnol. 20, 1067–1068.
Gille, H., Kowalski, J., Li, B., LeCouter, J., Moffat, B., Zioncheck, T.F., Pelletier, N., Ferrara, N., 2001. Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J. Biol. Chem. 276(5), 3222–3230.
Guglielmi, N., Hairer, E., 2001. Implementing Radau IIA methods for stiff delay differential equations. Computing 67(1), 1–12.
Holmes, M.J., Sleeman, B.D., 2000. A mathematical model of tumour angiogenesis incorporating cellular traction and viscoelastic effects. J. Theor. Biol. 202(2), 95–112.
Holmes, W.E., Lee, J., Kuang, W.J., Rice, G.C., Wood, W.I., 1991. Structure and functional expression of a human interleukin-8 receptor. Science 253(5025), 1278–1280.
Horuk, R., 1994. The interleukin-8-receptor family: from chemokines to malaria. Immunol. Today 15(4), 169–174.
Karl, E., Warner, K., Zeitlin, B., Kaneko, T., Wurtzel, L., Jin, T., Chang, J., Wang, S., Wang, C.Y., Strieter, R.M., Nunez, G., Polverini, P.J., Nör, J.E., 2005. Bcl-2 acts in a proangiogenic signaling pathway through nuclear factor-kappaB and CXC chemokines. Cancer Res. 65(12), 5063–5069.
Ke, L.D., Shi, Y.X., Im, S.A., Chen, X., Yung, W.K., 2000. The relevance of cell proliferation, vascular endothelial growth factor, and basic fibroblast growth factor production to angiogenesis and tumorigenicity in human glioma cell lines. Clin. Cancer Res. 6(6), 2562–2572.
Kim, K.J., Li, B., Winer, J., Armanini, M., Gillett, N., Phillips, H.S., Ferrara, N., 1993. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362(6423), 841–844.
Klintworth, G.K., 1973. The hamster cheek pouch: an experimental model of corneal vascularization. Am. J. Pathol. 73(3), 691–710.
Koch, A.E., Polverini, P.J., Kunkel, S.L., Harlow, L.A., DiPietro, L.A., Elner, V.M., Elner, S.G., Strieter, R.M., 1992. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 258(5089), 1798–1801.
Kuang, Y., Nagy, J.D., Elser, J.J., 2004. Biological stoichiometry of tumor dynamics. Discret. Contin. Dyn. Syst. Ser. B 4(1), 221–240.
Leung, D.W., Cachianes, G., Kuang, W.J., Goeddel, D.V., Ferrara, N., 1989. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246(4935), 1306–1309.
Levine, H.A., Sleeman, B.D., Nilsen-Hamilton, M., 2000. A mathematical model for the roles of pericytes and macrophages in the initiation of angiogenesis. I. The role of protease inhibitors in preventing angiogenesis. Math. Biosci. 168(1), 77–115.
Levine, H.A., Pamuk, S., Sleeman, B.D., Nilsen-Hamilton, M., 2001. Mathematical modeling of capillary formation and development in tumor angiogenesis: penetration into the stroma. Bull. Math. Biol. 63(5), 801–863.
Levine, H.A., Tucker, A.L., Nilsen-Hamilton, M., 2002. A mathematical model for the role of cell signal transduction in the initiation and inhibition of angiogenesis. Growth Factors 20(4), 155–175.
Mac Gabhann, F., Popel, A.S., 2004. Model of competitive binding of vascular endothelial growth factor and placental growth factor to VEGF receptors on endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 286(1), H153–H164.
McMahon, G., 2000. VEGF receptor signalling in tumor angiogenesis. Oncologist 5, 3–10.
Maher, J.J., 1995. Rat hepatocytes and Kupffer cells interact to produce interleukin-8 (CINC) in the setting of ethanol. Am. J. Physiol. 269(4 Pt 1), G518–G523.
Mukaida, N., 2003. Pathophysiological roles of interleukin-8/CXCL8 in pulmonary diseases. Am. J. Physiol. Lung Cell. Mol. Physiol. 284(4), L566–L577.
Nagy, J.D., 2004. Competition and natural selection in a mathematical model of cancer. Bull. Math. Biol. 66(4), 663–687.
Nguyen, M., Shing, Y., Folkman, J., 1994. Quantitation of angiogenesis and antiangiogenesis in the chick embryo chorioallantoic membrane. Microvasc. Res. 47(1), 31–40.
Nör, J.E., Christensen, J., Mooney, D.J., Polverini, P.J., 1999. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am. J. Pathol. 154(2), 375–384.
Nör, J.E., Christensen, J., Liu, J., Peters, M., Mooney, D.J., Strieter, R.M., Polverini, P.J., 2001a. Up-Regulation of Bcl-2 in microvascular endothelial cells enhances intratumoral angiogenesis and accelerates tumor growth. Cancer Res. 61(5), 2183–2188.
Nör, J.E., Peters, M.C., Christensen, J.B., Sutorik, M.M., Linn, S., Khan, M.K., Addison, C.L., Mooney, D.J., Polverini, P.J., 2001b. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab. Invest. 81(4), 453–463.
Norrby, K., 1998. Microvascular density in terms of number and length of microvessel segments per unit tissue volume in mammalian angiogenesis. Microvasc. Res. 55(1), 43–53.
Ohta, M., Kitadai, Y., Tanaka, S., Yoshihara, M., Yasui, W., Mukaida, N., Haruma, K., Chayama, K., 2002. Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human esophageal squamous cell carcinomas. Int. J. Cancer 102(3), 220–224.
Oikawa, T., Sasaki, M., Inose, M., 1997. Effect of cytogenin, a novel microbial product, on embryonic and tumor cell induced angiogenic responses in vivo. Anticancer Res. 17(3C), 1881–1886.
Patan, S., 2000. Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J. Neurooncol. 50(1–2), 1–15.
Pepper, M.S., Ferrara, N., Orci, L., Montesano, R., 1992. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem. Biophys. Res. Commun. 189(2), 824–831.
Pettet, G.J., Byrne, H.M., McElwain, D.L., Norbury, J., 1996a. A model of wound-healing angiogenesis in soft tissue. Math. Biosci. 136(1), 35–63.
Pettet, G., Chaplain, M.A., McElwain, D.L., Byrne, H.M., 1996b. On the role of angiogenesis in wound healing. Proc. Biol. Sci. 263(1376), 1487–1493.
Plank, M.J., Sleeman, B.D., 2003. A reinforced random walk model of tumour angiogenesis and anti-angiogenic strategies. Math. Med. Biol. 20(2), 135–181.
Plank, M.J., Sleeman, B.D., Jones, P.F., 2004. A mathematical model of tumour angiogenesis, regulated by vascular endothelial growth factor and the angiopoietins. J. Theor. Biol. 229(4), 435–454.
Pradeep, C.R., Sunila, E.S., Kuttan, G., 2005. Expression of vascular endothelial growth factor (VEGF) and VEGF receptors in tumor angiogenesis and malignancies. Integr. Cancer Ther. 4(4), 315–321.
Ribatti, D., Vacca, A., Roncali, L., Dammacco, F., 1996. The chick embryo chorioallantoic membrane as a model for in vivo research on angiogenesis. Int. J. Dev. Biol. 40(6), 1189–1197.
Ruhrberg, C., Gerhardt, H., Golding, M., Watson, R., Ioannidou, S., Fujisawa, H., Betsholtz, C., Shima, D.T., 2002. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 16(20), 2684–2698.
Salcedo, R., Ponce, M.L., Young, H.A., Wasserman, K., Ward, J.M., Kleinman, H.K., Oppenheim, J.J., Murphy, W.J., 2000. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood 96(1), 34–40.
Samanta, A.K., Oppenheim, J.J., Matsushima, K., 1989. Identification and characterization of specific receptors for monocyte-derived neutrophil chemotactic factor (MDNCF) on human neutrophils. J. Exp. Med. 169(3), 1185–1189.
Serini, G., Ambrosi, D., Giraudo, E., Gamba, A., Preziosi, L., Bussolino, F., 2003. Modeling the early stages of vascular network assembly. EMBO J. 22(8), 1771–1779.
Shweiki, D., Neeman, M., Itin, A., Keshet, E., 1995. Induction of vascular endothelial growth factor expression by hypoxia and by glucose deficiency in multicell spheroids: Implications for tumor angiogenesis. Proc. Natl. Acad. Sci. USA 92(3), 768–772.
Siemeister, G., Schirner, M., Reusch, P., Barleon, B., Marme, D., Martiny-Baron, G., 1998. An antagonistic vascular endothelial growth factor (VEGF) variant inhibits VEGF-stimulated receptor autophosphorylation and proliferation of human endothelial cells. Proc. Natl. Acad. Sci. USA 95(8), 4625–4629.
Smith, D.R., Polverini, P.J., Kunkel, S.L., Orringer, M.B., Whyte, R.I., Burdick, M.D., Wilke, C.A., Strieter, R.M., 1994. Inhibition of interleukin 8 attenuates angiogenesis in bronchogenic carcinoma. J. Exp. Med. 179(5), 1409–1415.
Spyridopoulos, I., Brogi, E., Kearney, M., Sullivan, A.B., Cetrulo, C., Isner, J.M., Losordo, D.W., 1997. Vascular endothelial growth factor inhibits endothelial cell apoptosis induced by tumor necrosis factor-alpha: balance between growth and death signals. J. Mol. Cell. Cardiol. 29(5), 1321–1330.
Stewart, M., Turley, H., Cook, N., Pezzella, F., Pillai, G., Ogilvie, D., Cartlidge, S., Paterson, D., Copley, C., Kendrew, J., Barnes, C., Harris, A.L., Gatter, K.C., 2003. The angiogenic receptor KDR is widely distributed in human tissues and tumours and relocates intracellularly on phosphorylation. An immunohistochemical study. Histopathology 43(1), 33–39.
Strieter, R.M., Kunkel, S.L., Elner, V.M., Martonyi, C.L., Koch, A.E., Polverini, P.J., Elner, S.G., 1992. Interleukin-8. A corneal factor that induces neovascularization. Am. J. Pathol. 141(6), 1279–1284.
Tee, D., DiStefano, J., 2004. Simulation of tumor-induced angiogenesis and its response to anti-angiogenic drug treatment: mode of drug delivery and clearance rate dependencies. J. Cancer Res. Clinical Oncol. 130(1), 15–24.
Terranova, V.P., DiFlorio, R., Lyall, R.M., Hic, S., Friesel, R., Maciag, T., 1985. Human endothelial cells are chemotactic to endothelial cell growth factor and heparin. J. Cell. Biol. 101(6), 2330–2334.
Trettel, F., Di Bartolomeo, S., Lauro, C., Catalano, M., Ciotti, M.T., Limatola, C., 2003. Ligand-independent CXCR2 dimerization. J. Biol. Chem. 278(42), 40980–40988.
Ueno, H., Li, J.J., Masuda, S., Qi, Z., Yamamoto, H., Takeshita, A., 1997. Adenovirus-mediated expression of the secreted form of basic fibroblast growth factor (FGF-2) induces cellular proliferation and angiogenesis in vivo. Arterioscler. Thromb. Vasc. Biol. 17(11), 2453–2460.
Wang, D., Lehman, R.E., Donner, D.B., Matli, M.R., Warren, R.S., Welton, M.L., 2002. Expression and endocytosis of VEGF and its receptors in human colonic vascular endothelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 282(6), G1088–G1096.
Ward, J.P., King, J.R., 1999. Mathematical modelling of avascular-tumour growth. II: Modelling growth saturation. IMA J. Math. Appl. Med. Biol. 16(2), 171–211.
Wilson, S., Wilkinson, G., Milligan, G., 2005. The CXCR1 and CXCR2 receptors form constitutive homo- and heterodimers selectively and with equal apparent affinities. J. Biol. Chem. 280(31), 28663–28674.
Yonekura, K., Basaki, Y., Chikahisa, L., 1999. UFT and its metabolites inhibit the angiogenesis induced by murine renal cell carcinoma, as determined by a dorsal air sac assay in mice. Clin. Cancer Res. 5(8), 2185–2191.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jain, H.V., Nör, J.E. & Jackson, T.L. Modeling the VEGF–Bcl-2–CXCL8 Pathway in Intratumoral Agiogenesis. Bull. Math. Biol. 70, 89–117 (2008). https://doi.org/10.1007/s11538-007-9242-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-007-9242-9