Abstract
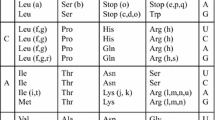
We develop here an analytical evolutionary model based on a trinucleotide mutation matrix 64× 64 with nine substitution parameters associated with the three types of substitutions in the three trinucleotide sites. It generalizes the previous models based on the nucleotide mutation matrices 4× 4 and the trinucleotide mutation matrix 64× 64 with three and six parameters. It determines at some time t the exact occurrence probabilities of trinucleotides mutating randomly according to these nine substitution parameters. An application of this model allows an evolutionary study of the common circular code \(\mathcal{C}\) of eukaryotes and prokaryotes and its 12 coded amino acids. The main property of this code \(\mathcal{C}\) is the retrieval of the reading frames in genes, both locally, i.e. anywhere in genes and in particular without a start codon, and automatically with a window of a few nucleotides. However, since its identification in 1996, amino acid information coded by \(\mathcal{C}\) has never been studied. Very unexpectedly, this evolutionary model demonstrates that random substitutions in this code \(\mathcal{C}\) and with particular values for the nine substitutions parameters retrieve after a certain time of evolution a frequency distribution of these 12 amino acids very close to the one coded by the actual genes.
Similar content being viewed by others
References
Akashi, H., Eyre-Walker, A., 1998. Translational selection and molecular evolution. Curr. Opin. Genet. Dev. 8, 688–693.
Antezana, M.A., Kreitman, M., 1999. The nonrandom location of synonymous codons suggests that reading frame-independent forces have patterned codon preferences. J. Mol. Evol. 49, 36–43.
Arquès, D.G., Fallot, J.-P., Michel, C.J., 1998. An evolutionary analytical model of a complementary circular code simulating the protein coding genes, the 5′ and 3′ regions. Bull. Math. Biol. 60, 163–194.
Arquès, D.G., Michel, C.J., 1996. A complementary circular code in the protein coding genes. J. Theor. Biol. 182, 45–58.
Béal, M.-P., 1993. Codage Symbolique. Masson, Paris.
Berg, O.G., Silva, P.J.N., 1997. Codon bias in Escherichia coli: The influence of codon context on mutation and selection. Nucleic Acids Res. 25, 1397–1404.
Berstel, J., Perrin, D., 1985. Theory of Codes. Academic Press, New York.
Bulmer, M., 1991. The selection-mutation-drift theory of synonymous codon usage. Genetics 129, 897–907.
Campbell, A., Mrázek, J., Karlin, S., 1999. Genomic signature comparisons among prokaryote, plasmid, and mitochondrial DNA. Proc. Natl. Acad. Sci. U.S.A. 96, 9184–9189.
Crick, F.H.C., Brenner, S., Klug, A., Pieczenik, G., 1976. A speculation on the origin of protein synthesis. Orig. Life 7, 389–397.
Crick, F.H.C., Griffith, J.S., Orgel, L.E., 1957. Codes without commas. Proc. Natl. Acad. Sci. 43, 416–421.
Fedorov, A., Saxonov, S., Gilbert, W., 2002. Regularities of context-dependent codon bias in eukaryotic genes. Nucleic Acids Res. 30, 1192–1197.
Grantham, R., Gautier, C., Gouy, M., Mercier, R., Pave, A., 1980. Codon catalog usage and the genome hypothesis. Nucleic Acids Res. 8, r49–r62.
Grantham, R., Gautier, C., Gouy, M., Jacobzone, M., Mercier, R., 1981. Codon catalog usage is a genome strategy modulated for gene expressivity. Nucleic Acids Res. 9, r43–r74.
Eigen, M., Schuster, P., 1978. The hypercycle. A principle of natural self-organization. Part C: The realistic hypercycle. Naturwissenschaften 65, 341–369.
Ermolaeva, M.D., 2001. Synonymous codon usage in bacteria. Curr. Issues Mol. Biol. Oct. 3, 91–97.
Frey, G., Michel, C.J., 2006. An analytical model of gene evolution with 6 mutation parameters: An application to archaeal circular codes. J. Comput. Biol. Chem. 30, 1–11.
Ikemura, T., 1985. Codon usage and tRNA content in unicellular and multicellular organisms. Mol. Biol. Evol. 2, 12–34.
Jukes, T.H., Bhushan, V., 1986. Silent nucleotide substitutions and G+C content of some mitochondrial and bacterial genes. J. Mol. Evol. 24, 39–44.
Jukes, T.H., Cantor, C.R., 1969. Evolution of protein molecules. In: Munro, H.N. (Ed.), Mammalian Protein Metabolism. Academic Press, New York, 21–132.
Kimura, M., 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16, 111–120.
Koch, A.J., Lehmann, J., 1997. About a symmetry of the genetic code. J. Theor. Biol. 189, 171–174.
Konu, O., Li, M.D., 2002. Correlations between mRNA expression levels and GC contents of coding and untranslated regions of genes in rodents. J. Mol. Evol. 54, 35–41.
Krakauer, D.C., Jansen, A.A., 2002. Red Queen Dynamics of protein Translation. J. Theor. Biol. 218, 97–109.
Lacan, J., Michel, C.J., 2001. Analysis of a circular code model. J. Theor. Biol. 213, 159–170.
Lange, K., 2005. Applied Probability, Springer-Verlag, New York.
Llopart, A., Aguade, M., 2000. Nucleotide polymorphism at the RpII215 gene in Drosophila subobscura: Weak selection on synonymous mutations. Genetics 155, 1245–1252.
Nirenberg, M.W., Matthaei, J.H., 1961. The dependance of cell-free protein synthesis in E. coli upon naturally occurring or synthetic polyribonucleotides. Proc. Natl. Acad. Sci. 47, 1588–1602.
Ochman, H., 2003. Neutral mutations and neutral substitutions in bacterial genomes. Mol. Biol. Evol. 20, 2091–2096.
Rogozin, I.B., Malyarchuk, B.A., Pavlov, Y.I., Milanesi, L., 2005. From context-dependance of mutations to molecular mechanisms of mutagenesis. Pac Symp. Biocomput., 409–420.
Rosenberg, M.S., Subramanian, S., Kumar, S., 2003. Patterns of transitional mutation biases within and among mammalian genomes. Mol. Biol. Evol. 20, 988–993.
Sharp, P.M., Bailes, E., Grocock, R.J., Peden, J.F., Sockett, R.E., 2005. Variation in the strength of selected codon usage bias among bacteria. Nucleic Acids Res. 33, 1141–1153.
Sharp, P.M., Matassi, G., 1994. Codon usage and genome evolution. Curr. Opin. Genet. Dev. 4, 851–860.
Shpaer, E.G., 1986. Constraints on codon context in Escherichia coli genes. Their possible role in modulating the efficiency of translation. J. Mol. Biol. 188, 555–564.
Smith, N.G.C., Eyre-Walker, A., 2001. Synonymous codon bias is not caused by mutation bias in G+C-rich genes in humans. Mol. Biol. Evol. 18, 982–986.
Yarus, M., Folley, L.S., 1984. Sense codons are found in specific contexts. J. Mol. Biol. 182, 529–540.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Michel, C.J. An Analytical Model of Gene Evolution with 9 Mutation Parameters: An Application to the Amino Acids Coded by the Common Circular Code. Bull. Math. Biol. 69, 677–698 (2007). https://doi.org/10.1007/s11538-006-9147-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-006-9147-z