Abstract
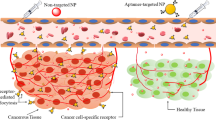
Conventional delivery of chemotherapeutic agents leads to multiple systemic side effects and toxicity, limiting the doses that can be used. The development of targeted therapies to selectively deliver anti-cancer agents to tumor cells without damaging neighboring unaffected cells would lead to higher effective local doses and improved response rates. Aptamers are single-stranded oligonucleotides that bind to target molecules with both high affinity and high specificity. The high specificity exhibited by aptamers promotes localization and uptake by specific cell populations, such as tumor cells, and their conjugation to anti-cancer drugs has been explored for targeted therapy. Advancements in the development of polymeric nanoparticles allow anti-cancer drugs to be encapsulated in protective nonreactive shells for controlled drug delivery with reduced toxicity. The conjugation of aptamers to nanoparticle-based therapeutics may further enhance direct targeting and personalized medicine. Here we present how the combinatorial use of aptamer and nanoparticle technologies has the potential to develop “smart bombs” for targeted cancer treatment, highlighting recent pre-clinical studies demonstrating efficacy for the direct targeting to particular tumor cell populations. However, despite these pre-clinical promising results, there has been little progress in moving this technology to the bedside.
Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65(1):5–29. doi:10.3322/caac.21254
Chen AC, Migliaccio I, Rimawi M, Lopez-Tarruella S, Creighton CJ, Massarweh S, Huang C, Wang YC, Batra SK, Gutierrez MC, Osborne CK, Schiff R (2012) Upregulation of mucin4 in ER-positive/HER2-overexpressing breast cancer xenografts with acquired resistance to endocrine and HER2-targeted therapies. Breast Cancer Res Treat 134(2):583–593. doi:10.1007/s10549-012-2082-9
Brody EN, Gold L (2000) Aptamers as therapeutic and diagnostic agents. J Biotechnol 74(1):5–13
Datta J, Xu S, Rosemblit C, Smith JB, Cintolo JA, Powell D Jr, Czerniecki BJ (2015) CD4+ T-helper type 1 cytokines and trastuzumab facilitate CD8+ T-cell targeting of HER-2/neu-expressing cancers. Cancer Immunol Res. doi:10.1158/2326-6066.CIR-14-0208
Meyer C, Hahn U, Rentmeister A (2011) Cell-specific aptamers as emerging therapeutics. J Nucleic Acids 2011:904750. doi:10.4061/2011/904750
Dausse E, Da Rocha GS, Toulme JJ (2009) Aptamers: a new class of oligonucleotides in the drug discovery pipeline? Curr Opin Pharmacol 9(5):602–607. doi:10.1016/j.coph.2009.07.006
Jayasena SD (1999) Aptamers: an emerging class of molecules that rival antibodies in diagnostics. Clin Chem 45(9):1628–1650
Sharma A, Jain N, Sareen R (2013) Nanocarriers for diagnosis and targeting of breast cancer. BioMed Res Int 2013:960821. doi:10.1155/2013/960821
Hu M, Zhang K (2013) The application of aptamers in cancer research: an up-to-date review. Future Oncol 9(3):369–376. doi:10.2217/fon.12.201
Petros RA, DeSimone JM (2010) Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov 9(8):615–627. doi:10.1038/nrd2591
Muthu MS, Leong DT, Mei L, Feng SS (2014) Nanotheranostics—application and further development of nanomedicine strategies for advanced theranostics. Theranostics 4(6):660–677. doi:10.7150/thno.8698
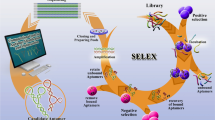
Fang X, Tan W (2010) Aptamers generated from cell-SELEX for molecular medicine: a chemical biology approach. Acc Chem Res 43(1):48–57. doi:10.1021/ar900101s
McKeague M, Derosa MC (2012) Challenges and opportunities for small molecule aptamer development. J Nucleic Acids 2012:748913. doi:10.1155/2012/748913
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249(4968):505–510
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346(6287):818–822. doi:10.1038/346818a0
Stoltenburg R, Reinemann C, Strehlitz B (2007) SELEX–a (r)evolutionary method to generate high-affinity nucleic acid ligands. Biomol Eng 24(4):381–403. doi:10.1016/j.bioeng.2007.06.001
Ara MN, Hyodo M, Ohga N, Hida K, Harashima H (2012) Development of a novel DNA aptamer ligand targeting to primary cultured tumor endothelial cells by a cell-based SELEX method. PLoS ONE 7(12):e50174. doi:10.1371/journal.pone.0050174
Xiao Z, Shangguan D, Cao Z, Fang X, Tan W (2008) Cell-specific internalization study of an aptamer from whole cell selection. Chemistry 14(6):1769–1775. doi:10.1002/chem.200701330
Xiao Z, Levy-Nissenbaum E, Alexis F, Luptak A, Teply BA, Chan JM, Shi J, Digga E, Cheng J, Langer R, Farokhzad OC (2012) Engineering of targeted nanoparticles for cancer therapy using internalizing aptamers isolated by cell-uptake selection. ACS Nano 6(1):696–704. doi:10.1021/nn204165v
Van Simaeys D, Lopez-Colon D, Sefah K, Sutphen R, Jimenez E, Tan W (2010) Study of the molecular recognition of aptamers selected through ovarian cancer cell-SELEX. PLoS ONE 5(11):e13770. doi:10.1371/journal.pone.0013770
Daniels DA, Chen H, Hicke BJ, Swiderek KM, Gold L (2003) A tenascin-C aptamer identified by tumor cell SELEX: systematic evolution of ligands by exponential enrichment. Proc Natl Acad Sci USA 100(26):15416–15421. doi:10.1073/pnas.2136683100
Tatarinova O, Tsvetkov V, Basmanov D, Barinov N, Smirnov I, Timofeev E, Kaluzhny D, Chuvilin A, Klinov D, Varizhuk A, Pozmogova G (2014) Comparison of the ‘chemical’ and ‘structural’ approaches to the optimization of the thrombin-binding aptamer. PLoS ONE 9(2):e89383. doi:10.1371/journal.pone.0089383
Chushak Y, Stone MO (2009) In silico selection of RNA aptamers. Nucleic Acids Res 37(12):e87. doi:10.1093/nar/gkp408
Zhu G, Ye M, Donovan MJ, Song E, Zhao Z, Tan W (2012) Nucleic acid aptamers: an emerging frontier in cancer therapy. Chem Commun (Camb) 48(85):10472–10480. doi:10.1039/c2cc35042d
Bazak R, Houri M, El Achy S, Kamel S, Refaat T (2014) Cancer active targeting by nanoparticles: a comprehensive review of literature. J Cancer Res Clin Oncol. doi:10.1007/s00432-014-1767-3
Esposito CL, Catuogno S, de Franciscis V, Cerchia L (2011) New insight into clinical development of nucleic acid aptamers. Discov Med 11(61):487–496
Zhu J, Huang H, Dong S, Ge L, Zhang Y (2014) Progress in aptamer-mediated drug delivery vehicles for cancer targeting and its implications in addressing chemotherapeutic challenges. Theranostics 4(9):931–944. doi:10.7150/thno.9663
Shum KT, Zhou J, Rossi JJ (2013) Nucleic acid aptamers as potential therapeutic and diagnostic agents for lymphoma. J Cancer Ther 4(4):872–890. doi:10.4236/jct.2013.44099
Healy JM, Lewis SD, Kurz M, Boomer RM, Thompson KM, Wilson C, McCauley TG (2004) Pharmacokinetics and biodistribution of novel aptamer compositions. Pharm Res 21(12):2234–2246
Kanwar JR, Roy K, Kanwar RK (2011) Chimeric aptamers in cancer cell-targeted drug delivery. Crit Rev Biochem Mol Biol 46(6):459–477. doi:10.3109/10409238.2011.614592
Lee JH, Yigit MV, Mazumdar D, Lu Y (2010) Molecular diagnostic and drug delivery agents based on aptamer-nanomaterial conjugates. Adv Drug Deliv Rev 62(6):592–605. doi:10.1016/j.addr.2010.03.003
Tan W, Wang H, Chen Y, Zhang X, Zhu H, Yang C, Yang R, Liu C (2011) Molecular aptamers for drug delivery. Trends Biotechnol 29(12):634–640. doi:10.1016/j.tibtech.2011.06.009
Zhang Y, Hong H, Cai W (2011) Tumor-targeted drug delivery with aptamers. Curr Med Chem 18(27):4185–4194
Talekar M, Kendall J, Denny W, Garg S (2011) Targeting of nanoparticles in cancer: drug delivery and diagnostics. Anti-Cancer Drugs 22(10):949–962. doi:10.1097/CAD.0b013e32834a4554
Keefe AD, Cload ST (2008) SELEX with modified nucleotides. Curr Opin Chem Biol 12(4):448–456. doi:10.1016/j.cbpa.2008.06.028
Burmeister PE, Lewis SD, Silva RF, Preiss JR, Horwitz LR, Pendergrast PS, McCauley TG, Kurz JC, Epstein DM, Wilson C, Keefe AD (2005) Direct in vitro selection of a 2′-O-methyl aptamer to VEGF. Chem Biol 12(1):25–33. doi:10.1016/j.chembiol.2004.10.017
Jellinek D, Green LS, Bell C, Lynott CK, Gill N, Vargeese C, Kirschenheuter G, McGee DP, Abesinghe P, Pieken WA et al (1995) Potent 2′-amino-2′-deoxypyrimidine RNA inhibitors of basic fibroblast growth factor. Biochemistry 34(36):11363–11372
Lin Y, Nieuwlandt D, Magallanez A, Feistner B, Jayasena SD (1996) High-affinity and specific recognition of human thyroid stimulating hormone (hTSH) by in vitro-selected 2′-amino-modified RNA. Nucleic Acids Res 24(17):3407–3414
Proske D, Gilch S, Wopfner F, Schatzl HM, Winnacker EL, Famulok M (2002) Prion-protein-specific aptamer reduces PrPSc formation. Chembiochem 3(8):717–725. doi:10.1002/1439-7633(20020802)3:8<717::AID-CBIC717>3.0.CO;2-C
Lin Y, Qiu Q, Gill SC, Jayasena SD (1994) Modified RNA sequence pools for in vitro selection. Nucleic Acids Res 22(24):5229–5234
Kato Y, Minakawa N, Komatsu Y, Kamiya H, Ogawa N, Harashima H, Matsuda A (2005) New NTP analogs: the synthesis of 4′-thioUTP and 4′-thioCTP and their utility for SELEX. Nucleic Acids Res 33(9):2942–2951. doi:10.1093/nar/gki578
Kang J, Lee MS, Copland JA 3rd, Luxon BA, Gorenstein DG (2008) Combinatorial selection of a single stranded DNA thioaptamer targeting TGF-beta1 protein. Bioorg Med Chem Lett 18(6):1835–1839. doi:10.1016/j.bmcl.2008.02.023
Weiss S, Proske D, Neumann M, Groschup MH, Kretzschmar HA, Famulok M, Winnacker EL (1997) RNA aptamers specifically interact with the prion protein PrP. J Virol 71(11):8790–8797
Porter KW, Briley JD, Shaw BR (1997) Direct PCR sequencing with boronated nucleotides. Nucleic Acids Res 25(8):1611–1617
Chakravarthy U, Adamis AP, Cunningham ET Jr, Goldbaum M, Guyer DR, Katz B, Patel M (2006) Year 2 efficacy results of 2 randomized controlled clinical trials of pegaptanib for neovascular age-related macular degeneration. Ophthalmology 113(9):1508 e1501–1525. doi:10.1016/j.ophtha.2006.02.064
Obubuafo A, Balamurugan S, Shadpour H, Spivak D, McCarley RL, Soper SA (2008) Poly(methyl methacrylate) microchip affinity capillary gel electrophoresis of aptamer-protein complexes for the analysis of thrombin in plasma. Electrophoresis 29(16):3436–3445. doi:10.1002/elps.200700854
Gordon EM, Hall FL (2010) Rexin-G, a targeted genetic medicine for cancer. Expert Opin Biol Ther 10(5):819–832. doi:10.1517/14712598.2010.481666
He K, Porter KW, Hasan A, Briley J, Shaw BR (1999) Synthesis of 5-substituted 2′-deoxycytidine 5′-(alpha-P-borano)triphosphates, their incorporationinto DNA and effects on exonuclease. Nucleic Acids Res 27(8):1788–1794
Masud MM, Kuwahara M, Ozaki H, Sawai H (2004) Sialyllactose-binding modified DNA aptamer bearing additional functionality by SELEX. Bioorg Med Chem 12(5):1111–1120. doi:10.1016/j.bmc.2003.12.009
Kuwahara M, Sugimoto N (2010) Molecular evolution of functional nucleic acids with chemical modifications. Molecules 15(8):5423–5444. doi:10.3390/molecules15085423
Bundgaard Jensen TB, Pasternak A, Madsen AS, Petersen M, Wengel J (2011) Synthesis and structural characterization of 2 ′-fluoro-alpha-L-RNA-modified oligonucleotides. Chembiochem 12(12):1903–1910. doi:10.1002/cbic.201100161
Layzer JM, McCaffrey AP, Tanner AK, Huang Z, Kay MA, Sullenger BA (2004) In vivo activity of nuclease-resistant siRNAs. RNA 10(5):766–771
Schmidt KS, Borkowski S, Kurreck J, Stephens AW, Bald R, Hecht M, Friebe M, Dinkelborg L, Erdmann VA (2004) Application of locked nucleic acids to improve aptamer in vivo stability and targeting function. Nucleic Acids Res 32(19):5757–5765. doi:10.1093/nar/gkh862
Kuwahara M, Obika S (2013) In vitro selection of BNA (LNA) aptamers. Artif DNA PNA XNA 4(2):39–48. doi:10.4161/adna.25786
Singh SK, Kumar R, Wengel J (1998) Synthesis of novel Bicyclo[2.2.1] ribonucleosides: 2′-Amino- and 2′-thio-LNA monomeric nucleosides. J Org Chem 63(18):6078–6079
Petersen M, Bondensgaard K, Wengel J, Jacobsen JP (2002) Locked nucleic acid (LNA) recognition of RNA: NMR solution structures of LNA: RNA hybrids. J Am Chem Soc 124(21):5974–5982. doi:10.1021/Ja012288d
Yang X, Fennewald S, Luxon BA, Aronson J, Herzog NK, Gorenstein DG (1999) Aptamers containing thymidine 3′-O-phosphorodithioates: synthesis and binding to nuclear factor-kappaB. Bioorg Med Chem Lett 9(23):3357–3362
Sundaram P, Kurniawan H, Byrne ME, Wower J (2012) Therapeutic RNA aptamers in clinical trials. Eur J Pharm Sci 48(1–2):259–271. doi:10.1016/j.ejps.2012.10.014
Jo N, Mailhos C, Ju M, Cheung E, Bradley J, Nishijima K, Robinson GS, Adamis AP, Shima DT (2006) Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am J Pathol 168(6):2036–2053. doi:10.2353/ajpath.2006.050588
Chelliserrykattil J, Ellington AD (2004) Evolution of a T7 RNA polymerase variant that transcribes 2′-O-methyl RNA. Nat Biotechnol 22(9):1155–1160. doi:10.1038/nbt1001
Fa M, Radeghieri A, Henry AA, Romesberg FE (2004) Expanding the substrate repertoire of a DNA polymerase by directed evolution. J Am Chem Soc 126(6):1748–1754. doi:10.1021/ja038525p
Sousa R, Padilla R (1995) A mutant T7 RNA polymerase as a DNA polymerase. EMBO J 14(18):4609–4621
Sheehan JP, Lan HC (1998) Phosphorothioate oligonucleotides inhibit the intrinsic tenase complex. Blood 92(5):1617–1625
Farman CA, Kornbrust DJ (2003) Oligodeoxynucleotide studies in primates: antisense and immune stimulatory indications. Toxicol Pathol 31[Suppl]:119–122
Henry SP, Bolte H, Auletta C, Kornbrust DJ (1997) Evaluation of the toxicity of ISIS 2302, a phosphorothioate oligonucleotide, in a four-week study in cynomolgus monkeys. Toxicology 120(2):145–155
Frazier KS (2015) Antisense oligonucleotide therapies: the promise and the challenges from a toxicologic pathologist’s perspective. Toxicol Pathol 43(1):78–89. doi:10.1177/0192623314551840
Swayze EE, Siwkowski AM, Wancewicz EV, Migawa MT, Wyrzykiewicz TK, Hung G, Monia BP, Bennett CF (2007) Antisense oligonucleotides containing locked nucleic acid improve potency but cause significant hepatotoxicity in animals. Nucleic Acids Res 35(2):687–700. doi:10.1093/nar/gkl1071
Hagedorn PH, Yakimov V, Ottosen S, Kammler S, Nielsen NF, Hog AM, Hedtjarn M, Meldgaard M, Moller MR, Orum H, Koch T, Lindow M (2013) Hepatotoxic potential of therapeutic oligonucleotides can be predicted from their sequence and modification pattern. Nucleic Acid Ther 23(5):302–310. doi:10.1089/nat.2013.0436
Barchet W, Wimmenauer V, Schlee M, Hartmann G (2008) Accessing the therapeutic potential of immunostimulatory nucleic acids. Curr Opin Immunol 20(4):389–395. doi:10.1016/j.coi.2008.07.007
Bouchard PR, Hutabarat RM, Thompson KM (2010) Discovery and development of therapeutic aptamers. Annu Rev Pharmacol Toxicol 50:237–257. doi:10.1146/annurev.pharmtox.010909.105547
Henry SP, Giclas PC, Leeds J, Pangburn M, Auletta C, Levin AA, Kornbrust DJ (1997) Activation of the alternative pathway of complement by a phosphorothioate oligonucleotide: potential mechanism of action. J Pharmacol Exp Ther 281(2):810–816
Henry SP, Monteith D, Levin AA (1997) Antisense oligonucleotide inhibitors for the treatment of cancer: 2. Toxicological properties of phosphorothioate oligodeoxynucleotides. Anticancer Drug Des 12(5):395–408
Huang YF, Shangguan D, Liu H, Phillips JA, Zhang X, Chen Y, Tan W (2009) Molecular assembly of an aptamer-drug conjugate for targeted drug delivery to tumor cells. Chembiochem 10(5):862–868. doi:10.1002/cbic.200800805
Buerger C, Groner B (2003) Bifunctional recombinant proteins in cancer therapy: cell penetrating peptide aptamers as inhibitors of growth factor signaling. J Cancer Res Clin Oncol 129(12):669–675. doi:10.1007/s00432-003-0489-8
Du KL, Mick R, Busch TM, Zhu TC, Finlay JC, Yu G, Yodh AG, Malkowicz SB, Smith D, Whittington R, Stripp D, Hahn SM (2006) Preliminary results of interstitial motexafin lutetium-mediated PDT for prostate cancer. Lasers Surg Med 38(5):427–434. doi:10.1002/lsm.20341
Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE et al (1994) Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 331(22):1480–1487. doi:10.1056/NEJM199412013312203
Kieran MW, Kalluri R, Cho YJ (2012) The VEGF pathway in cancer and disease: responses, resistance, and the path forward. Cold Spring Harb Perspect Med 2(12):a006593. doi:10.1101/cshperspect.a006593
Linger RM, Keating AK, Earp HS, Graham DK (2008) TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res 100:35–83. doi:10.1016/S0065-230X(08)00002-X
Verma A, Warner SL, Vankayalapati H, Bearss DJ, Sharma S (2011) Targeting Axl and Mer kinases in cancer. Mol Cancer Ther 10(10):1763–1773. doi:10.1158/1535-7163.MCT-11-0116
Hector A, Montgomery EA, Karikari C, Canto M, Dunbar KB, Wang JS, Feldmann G, Hong SM, Haffner MC, Meeker AK, Holland SJ, Yu J, Heckrodt TJ, Zhang J, Ding P, Goff D, Singh R, Roa JC, Marimuthu A, Riggins GJ, Eshleman JR, Nelkin BD, Pandey A, Maitra A (2010) The Axl receptor tyrosine kinase is an adverse prognostic factor and a therapeutic target in esophageal adenocarcinoma. Cancer Biol Ther 10(10):1009–1018. doi:10.4161/cbt.10.10.13248
Holland SJ, Pan A, Franci C, Hu Y, Chang B, Li W, Duan M, Torneros A, Yu J, Heckrodt TJ, Zhang J, Ding P, Apatira A, Chua J, Brandt R, Pine P, Goff D, Singh R, Payan DG, Hitoshi Y (2010) R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res 70(4):1544–1554. doi:10.1158/0008-5472.CAN-09-2997
Ye X, Li Y, Stawicki S, Couto S, Eastham-Anderson J, Kallop D, Weimer R, Wu Y, Pei L (2010) An anti-Axl monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies. Oncogene 29(38):5254–5264. doi:10.1038/onc.2010.268
Cerchia L, Esposito CL, Camorani S, Rienzo A, Stasio L, Insabato L, Affuso A, de Franciscis V (2012) Targeting Axl with an high-affinity inhibitory aptamer. Mol Ther 20(12):2291–2303. doi:10.1038/mt.2012.163
Jones AV, Cross NC (2004) Oncogenic derivatives of platelet-derived growth factor receptors. Cell Mol life Sci 61(23):2912–2923. doi:10.1007/s00018-004-4272-z
Strand J, Varasteh Z, Eriksson O, Abrahmsen L, Orlova A, Tolmachev V (2014) Gallium-68-labeled affibody molecule for PET imaging of PDGFRbeta expression in vivo. Mol Pharm 11(11):3957–3964. doi:10.1021/mp500284t
Camorani S, Esposito CL, Rienzo A, Catuogno S, Iaboni M, Condorelli G, de Franciscis V, Cerchia L (2014) Inhibition of receptor signaling and of glioblastoma-derived tumor growth by a novel PDGFRbeta aptamer. Mol Ther 22(4):828–841. doi:10.1038/mt.2013.300
Biesecker G, Dihel L, Enney K, Bendele RA (1999) Derivation of RNA aptamer inhibitors of human complement C5. Immunopharmacology 42(1–3):219–230
Ackroyd R, Kelty CJ, Brown NJ, Stephenson TJ, Stoddard CJ, Reed MW (2003) Eradication of dysplastic Barrett’s oesophagus using photodynamic therapy: long-term follow-up. Endoscopy 35(6):496–501. doi:10.1055/s-2003-39676
Cuenca RE, Allison RR, Sibata C, Downie GH (2004) Breast cancer with chest wall progression: treatment with photodynamic therapy. Ann Surg Oncol 11(3):322–327
Moore CM, Emberton M, Bown SG (2011) Photodynamic therapy for prostate cancer–an emerging approach for organ-confined disease. Lasers Surg Med 43(7):768–775. doi:10.1002/lsm.21104
Lamberti MJ, Vittar NB, Rivarola VA (2014) Breast cancer as photodynamic therapy target: enhanced therapeutic efficiency by overview of tumor complexity. World J Clin Oncol 5(5):901–907. doi:10.5306/wjco.v5.i5.901
Yano T, Hatogai K, Morimoto H, Yoda Y, Kaneko K (2014) Photodynamic therapy for esophageal cancer. Ann Transl Med 2(3):29. doi:10.3978/j.issn.2305-5839.2014.03.01
Tang Z, Shangguan D, Wang K, Shi H, Sefah K, Mallikratchy P, Chen HW, Li Y, Tan W (2007) Selection of aptamers for molecular recognition and characterization of cancer cells. Anal Chem 79(13):4900–4907. doi:10.1021/ac070189y
Mallikaratchy P, Tang Z, Tan W (2008) Cell specific aptamer-photosensitizer conjugates as a molecular tool in photodynamic therapy. ChemMedChem 3(3):425–428. doi:10.1002/cmdc.200700260
Shieh YA, Yang SJ, Wei MF, Shieh MJ (2010) Aptamer-based tumor-targeted drug delivery for photodynamic therapy. ACS Nano 4(3):1433–1442. doi:10.1021/nn901374b
Soundararajan S, Chen W, Spicer EK, Courtenay-Luck N, Fernandes DJ (2008) The nucleolin targeting aptamer AS1411 destabilizes Bcl-2 messenger RNA in human breast cancer cells. Cancer Res 68(7):2358–2365. doi:10.1158/0008-5472.CAN-07-5723
Liu Q, Xu L, Zhang X, Li N, Zheng J, Guan M, Fang X, Wang C, Shu C (2013) Enhanced photodynamic efficiency of an aptamer-guided fullerene photosensitizer toward tumor cells. Chem Asian J 8(10):2370–2376. doi:10.1002/asia.201300039
Ferreira CS, Cheung MC, Missailidis S, Bisland S, Gariepy J (2009) Phototoxic aptamers selectively enter and kill epithelial cancer cells. Nucleic Acids Res 37(3):866–876. doi:10.1093/nar/gkn967
Wang K, You M, Chen Y, Han D, Zhu Z, Huang J, Williams K, Yang CJ, Tan W (2011) Self-assembly of a bifunctional DNA carrier for drug delivery. Angew Chem Int Ed Engl 50(27):6098–6101. doi:10.1002/anie.201008053
Han D, Zhu G, Wu C, Zhu Z, Chen T, Zhang X, Tan W (2013) Engineering a cell-surface aptamer circuit for targeted and amplified photodynamic cancer therapy. ACS Nano 7(3):2312–2319. doi:10.1021/nn305484p
Gopinath SC, Kumar PK (2013) Aptamers that bind to the hemagglutinin of the recent pandemic influenza virus H1N1 and efficiently inhibit agglutination. Acta Biomater 9(11):8932–8941. doi:10.1016/j.actbio.2013.06.016
Musafia B, Oren-Banaroya R, Noiman S (2014) Designing anti-influenza aptamers: novel quantitative structure activity relationship approach gives insights into aptamer-virus interaction. PLoS ONE 9(5):e97696. doi:10.1371/journal.pone.0097696
Binning JM, Wang T, Luthra P, Shabman RS, Borek DM, Liu G, Xu W, Leung DW, Basler CF, Amarasinghe GK (2013) Development of RNA aptamers targeting Ebola virus VP35. Biochemistry 52(47):8406–8419. doi:10.1021/bi400704d
Liang HR, Hu GQ, Zhang T, Yang YJ, Zhao LL, Qi YL, Wang HL, Gao YW, Yang ST, Xia XZ (2012) Isolation of ssDNA aptamers that inhibit rabies virus. Int Immunopharmacol 14(3):341–347. doi:10.1016/j.intimp.2012.06.019
Gourronc FA, Rockey WM, Thiel WH, Giangrande PH, Klingelhutz AJ (2013) Identification of RNA aptamers that internalize into HPV-16 E6/E7 transformed tonsillar epithelial cells. Virology 446(1–2):325–333. doi:10.1016/j.virol.2013.08.015
Nicol C, Cesur O, Forrest S, Belyaeva TA, Bunka DH, Blair GE, Stonehouse NJ (2013) An RNA aptamer provides a novel approach for the induction of apoptosis by targeting the HPV16 E7 oncoprotein. PLoS ONE 8(5):e64781. doi:10.1371/journal.pone.0064781
Toscano-Garibay JD, Benitez-Hess ML, Alvarez-Salas LM (2011) Isolation and characterization of an RNA aptamer for the HPV-16 E7 oncoprotein. Arch Med Res 42(2):88–96. doi:10.1016/j.arcmed.2011.02.005
Nicol C, Bunka DH, Blair GE, Stonehouse NJ (2011) Effects of single nucleotide changes on the binding and activity of RNA aptamers to human papillomavirus 16 E7 oncoprotein. Biochem Biophys Res Commun 405(3):417–421. doi:10.1016/j.bbrc.2011.01.044
Zhu G, Meng L, Ye M, Yang L, Sefah K, O’Donoghue MB, Chen Y, Xiong X, Huang J, Song E, Tan W (2012) Self-assembled aptamer-based drug carriers for bispecific cytotoxicity to cancer cells. Chem Asian J 7(7):1630–1636. doi:10.1002/asia.201101060
Lupold SE, Hicke BJ, Lin Y, Coffey DS (2002) Identification and characterization of nuclease-stabilized RNA molecules that bind human prostate cancer cells via the prostate-specific membrane antigen. Cancer Res 62(14):4029–4033
Taghdisi SM, Danesh NM, Sarreshtehdar Emrani A, Tabrizian K, Zandkarimi M, Ramezani M, Abnous K (2013) Targeted delivery of epirubicin to cancer cells by PEGylated A10 aptamer. J Drug Target 21(8):739–744. doi:10.3109/1061186X.2013.812095
Taghdisi SM, Abnous K, Mosaffa F, Behravan J (2010) Targeted delivery of daunorubicin to T-cell acute lymphoblastic leukemia by aptamer. J Drug Target 18(4):277–281. doi:10.3109/10611860903434050
Willner D, Trail PA, Hofstead SJ, King HD, Lasch SJ, Braslawsky GR, Greenfield RS, Kaneko T, Firestone RA (1993) (6-Maleimidocaproyl)hydrazone of doxorubicin–a new derivative for the preparation of immunoconjugates of doxorubicin. Bioconjug Chem 4(6):521–527
Meng L, Yang L, Zhao X, Zhang L, Zhu H, Liu C, Tan W (2012) Targeted delivery of chemotherapy agents using a liver cancer-specific aptamer. PLoS ONE 7(4):e33434. doi:10.1371/journal.pone.0033434
Subramanian N, Raghunathan V, Kanwar JR, Kanwar RK, Elchuri SV, Khetan V, Krishnakumar S (2012) Target-specific delivery of doxorubicin to retinoblastoma using epithelial cell adhesion molecule aptamer. Mol Vis 18:2783–2795
Liu Z, Duan JH, Song YM, Ma J, Wang FD, Lu X, Yang XD (2012) Novel HER2 aptamer selectively delivers cytotoxic drug to HER2-positive breast cancer cells in vitro. J Transl Med 10:148. doi:10.1186/1479-5876-10-148
Hu Y, Duan J, Zhan Q, Wang F, Lu X, Yang XD (2012) Novel MUC1 aptamer selectively delivers cytotoxic agent to cancer cells in vitro. PLoS ONE 7(2):e31970. doi:10.1371/journal.pone.0031970
Vasir JK, Labhasetwar V (2007) Biodegradable nanoparticles for cytosolic delivery of therapeutics. Adv Drug Deliv Rev 59(8):718–728. doi:10.1016/j.addr.2007.06.003
Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Preat V (2012) PLGA-based nanoparticles: an overview of biomedical applications. J Control Release 161(2):505–522. doi:10.1016/j.jconrel.2012.01.043
Lee IH, An S, Yu MK, Kwon HK, Im SH, Jon S (2011) Targeted chemoimmunotherapy using drug-loaded aptamer-dendrimer bioconjugates. J Control Release 155(3):435–441. doi:10.1016/j.jconrel.2011.05.025
Wang G, Uludag H (2008) Recent developments in nanoparticle-based drug delivery and targeting systems with emphasis on protein-based nanoparticles. Expert Opin Drug Deliv 5(5):499–515. doi:10.1517/17425247.5.5.499
D’Addio SM, Prud’homme RK (2011) Controlling drug nanoparticle formation by rapid precipitation. Adv Drug Deliv Rev 63(6):417–426. doi:10.1016/j.addr.2011.04.005
Letchford K, Burt H (2007) A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: micelles, nanospheres, nanocapsules and polymersomes. Eur J Pharm Biopharm 65(3):259–269. doi:10.1016/j.ejpb.2006.11.009
Wu C, Han D, Chen T, Peng L, Zhu G, You M, Qiu L, Sefah K, Zhang X, Tan W (2013) Building a multifunctional aptamer-based DNA nanoassembly for targeted cancer therapy. J Am Chem Soc 135(49):18644–18650. doi:10.1021/ja4094617
Zhu G, Hu R, Zhao Z, Chen Z, Zhang X, Tan W (2013) Noncanonical self-assembly of multifunctional DNA nanoflowers for biomedical applications. J Am Chem Soc 135(44):16438–16445. doi:10.1021/ja406115e
Smitha SL, Gopchandran KG (2013) Surface enhanced Raman scattering, antibacterial and antifungal active triangular gold nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc 102:114–119. doi:10.1016/j.saa.2012.09.055
Chong CS, Cao M, Wong WW, Fischer KP, Addison WR, Kwon GS, Tyrrell DL, Samuel J (2005) Enhancement of T helper type 1 immune responses against hepatitis B virus core antigen by PLGA nanoparticle vaccine delivery. J Control Release 102(1):85–99. doi:10.1016/j.jconrel.2004.09.014
Werner ME, Karve S, Sukumar R, Cummings ND, Copp JA, Chen RC, Zhang T, Wang AZ (2011) Folate-targeted nanoparticle delivery of chemo- and radiotherapeutics for the treatment of ovarian cancer peritoneal metastasis. Biomaterials 32(33):8548–8554. doi:10.1016/j.biomaterials.2011.07.067
Dhar S, Gu FX, Langer R, Farokhzad OC, Lippard SJ (2008) Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles. Proc Natl Acad Sci USA 105(45):17356–17361. doi:10.1073/pnas.0809154105
Kumari A, Yadav SK, Yadav SC (2010) Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces 75(1):1–18. doi:10.1016/j.colsurfb.2009.09.001
Koziara JM, Whisman TR, Tseng MT, Mumper RJ (2006) In-vivo efficacy of novel paclitaxel nanoparticles in paclitaxel-resistant human colorectal tumors. J Control Release 112(3):312–319. doi:10.1016/j.jconrel.2006.03.001
Rouf MA, Vural I, Renoir JM, Hincal AA (2009) Development and characterization of liposomal formulations for rapamycin delivery and investigation of their antiproliferative effect on MCF7 cells. J Liposome Res 19(4):322–331. doi:10.3109/08982100902963043
Gabizon A, Catane R, Uziely B, Kaufman B, Safra T, Cohen R, Martin F, Huang A, Barenholz Y (1994) Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res 54(4):987–992
Gabizon A, Isacson R, Libson E, Kaufman B, Uziely B, Catane R, Ben-Dor CG, Rabello E, Cass Y, Peretz T et al (1994) Clinical studies of liposome-encapsulated doxorubicin. Acta Oncol 33(7):779–786
Gabizon AA (1994) Liposomal anthracyclines. Hematol Oncol Clin North Am 8(2):431–450
Schroeder A, Goldberg MS, Kastrup C, Wang Y, Jiang S, Joseph BJ, Levins CG, Kannan ST, Langer R, Anderson DG (2012) Remotely activated protein-producing nanoparticles. Nano Lett 12(6):2685–2689. doi:10.1021/nl2036047
Yang L, Zhang X, Ye M, Jiang J, Yang R, Fu T, Chen Y, Wang K, Liu C, Tan W (2011) Aptamer-conjugated nanomaterials and their applications. Adv Drug Deliv Rev 63(14–15):1361–1370. doi:10.1016/j.addr.2011.10.002
Rafiyath SM, Rasul M, Lee B, Wei G, Lamba G, Liu D (2012) Comparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: a meta-analysis. Exp. Hematol Oncol 1(1):10. doi:10.1186/2162-3619-1-10
Harris L, Batist G, Belt R, Rovira D, Navari R, Azarnia N, Welles L, Winer E (2002) Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer 94(1):25–36. doi:10.1002/cncr.10201
Hatakeyama H, Akita H, Harashima H (2013) The polyethyleneglycol dilemma: advantage and disadvantage of PEGylation of liposomes for systemic genes and nucleic acids delivery to tumors. Biol Pharm Bull 36(6):892–899
Qaddoumi MG, Ueda H, Yang J, Davda J, Labhasetwar V, Lee VH (2004) The characteristics and mechanisms of uptake of PLGA nanoparticles in rabbit conjunctival epithelial cell layers. Pharm Res 21(4):641–648
Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R (1994) Biodegradable long-circulating polymeric nanospheres. Science 263(5153):1600–1603
Bege N, Renette T, Jansch M, Reul R, Merkel O, Petersen H, Curdy C, Muller RH, Kissel T (2011) Biodegradable poly(ethylene carbonate) nanoparticles as a promising drug delivery system with “stealth” potential. Macromol Biosci 11(7):897–904. doi:10.1002/mabi.201000496
Feczko T, Toth J, Dosa G, Gyenis J (2011) Influence of process conditions on the mean size of PLGA nanoparticles. Chem Eng Process 50(8):846–853. doi:10.1016/j.cep.2011.05.006
Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, Black K, Sisti M, Brem S, Mohr G et al (1995) Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The polymer-brain tumor treatment group. Lancet 345(8956):1008–1012
Westphal M, Ram Z, Riddle V, Hilt D, Bortey E (2006) Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir (Wien) 148(3):269–275. doi:10.1007/s00701-005-0707-z, discussion 275
Ran Z, Sun Y, Chang B, Ren Q, Yang W (2013) Silica composite nanoparticles containing fluorescent solid core and mesoporous shell with different thickness as drug carrier. J Colloid Interface Sci 410:94–101. doi:10.1016/j.jcis.2013.08.015
Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI (2012) Mesoporous silica nanoparticles in biomedical applications. Chem Soc Rev 41(7):2590–2605. doi:10.1039/c1cs15246g
Wu X, Wu M, Zhao JX (2014) Recent development of silica nanoparticles as delivery vectors for cancer imaging and therapy. Nanomedicine 10(2):297–312. doi:10.1016/j.nano.2013.08.008
Kwon S, Singh RK, Perez RA, Abou Neel EA, Kim HW, Chrzanowski W (2013) Silica-based mesoporous nanoparticles for controlled drug delivery. J Tissue Eng 4:2041731413503357. doi:10.1177/2041731413503357
Lee JE, Lee N, Kim H, Kim J, Choi SH, Kim JH, Kim T, Song IC, Park SP, Moon WK, Hyeon T (2010) Uniform mesoporous dye-doped silica nanoparticles decorated with multiple magnetite nanocrystals for simultaneous enhanced magnetic resonance imaging, fluorescence imaging, and drug delivery. J Am Chem Soc 132(2):552–557. doi:10.1021/ja905793q
Singh N, Karambelkar A, Gu L, Lin K, Miller JS, Chen CS, Sailor MJ, Bhatia SN (2011) Bioresponsive mesoporous silica nanoparticles for triggered drug release. J Am Chem Soc 133(49):19582–19585. doi:10.1021/ja206998x
Jia L, Shen J, Li Z, Zhang D, Zhang Q, Liu G, Zheng D, Tian X (2013) In vitro and in vivo evaluation of paclitaxel-loaded mesoporous silica nanoparticles with three pore sizes. Int J Pharm 445(1–2):12–19. doi:10.1016/j.ijpharm.2013.01.058
Chen C, Geng J, Pu F, Yang X, Ren J, Qu X (2011) Polyvalent nucleic acid/mesoporous silica nanoparticle conjugates: dual stimuli-responsive vehicles for intracellular drug delivery. Angew Chem Int Ed Engl 50(4):882–886. doi:10.1002/anie.201005471
Meng H, Xue M, Xia T, Zhao YL, Tamanoi F, Stoddart JF, Zink JI, Nel AE (2010) Autonomous in vitro anticancer drug release from mesoporous silica nanoparticles by pH-sensitive nanovalves. J Am Chem Soc 132(36):12690–12697. doi:10.1021/ja104501a
Wu C, Chen C, Lai J, Chen J, Mu X, Zheng J, Zhao Y (2008) Molecule-scale controlled-release system based on light-responsive silica nanoparticles. Chem Commun (Camb) 23:2662–2664. doi:10.1039/b804886j
El-Ghannam A, Ricci K, Malkawi A, Jahed K, Vedantham K, Wyan H, Allen LD, Dreau D (2010) A ceramic-based anticancer drug delivery system to treat breast cancer. J Mater Sci Mater Med 21(9):2701–2710. doi:10.1007/s10856-010-4121-6
Chen Z, Li X, He H, Ren Z, Liu Y, Wang J, Li Z, Shen G, Han G (2012) Mesoporous silica nanoparticles with manipulated microstructures for drug delivery. Colloids Surf B Biointerfaces 95:274–278. doi:10.1016/j.colsurfb.2012.03.012
Park JH, Gu L, von Maltzahn G, Ruoslahti E, Bhatia SN, Sailor MJ (2009) Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat Mater 8(4):331–336. doi:10.1038/nmat2398
Cai L, Chen ZZ, Chen MY, Tang HW, Pang DW (2013) MUC-1 aptamer-conjugated dye-doped silica nanoparticles for MCF-7 cells detection. Biomaterials 34(2):371–381. doi:10.1016/j.biomaterials.2012.09.084
Babu E, Mareeswaran PM, Rajagopal S (2013) Highly sensitive optical biosensor for thrombin based on structure switching aptamer-luminescent silica nanoparticles. J Fluoresc 23(1):137–146. doi:10.1007/s10895-012-1127-0
Hernandez FJ, Hernandez LI, Pinto A, Schafer T, Ozalp VC (2013) Targeting cancer cells with controlled release nanocapsules based on a single aptamer. Chem Commun (Camb) 49(13):1285–1287. doi:10.1039/c2cc37370j
Kim S, Ohulchanskyy TY, Bharali D, Chen Y, Pandey RK, Prasad PN (2009) Organically modified silica nanoparticles with intraparticle heavy-atom effect on the encapsulated photosensitizer for enhanced efficacy of photodynamic therapy. J Phys Chem C Nanomater Interfaces 113:12641–12644. doi:10.1021/jp900573s
Peng J, Zhao L, Zhu X, Sun Y, Feng W, Gao Y, Wang L, Li F (2013) Hollow silica nanoparticles loaded with hydrophobic phthalocyanine for near-infrared photodynamic and photothermal combination therapy. Biomaterials 34(32):7905–7912. doi:10.1016/j.biomaterials.2013.07.027
Yin J, He X, Wang K, Qing Z, Wu X, Shi H, Yang X (2012) One-step engineering of silver nanoclusters-aptamer assemblies as luminescent labels to target tumor cells. Nanoscale 4(1):110–112. doi:10.1039/c1nr11265a
Zhang JQ, Wang YS, Xue JH, He Y, Yang HX, Liang J, Shi LF, Xiao XL (2012) A gold nanoparticles-modified aptamer beacon for urinary adenosine detection based on structure-switching/fluorescence-“turning on” mechanism. J Pharm Biomed Anal 70:362–368. doi:10.1016/j.jpba.2012.05.032
Wang P, Song Y, Zhao Y, Fan A (2013) Hydroxylamine amplified gold nanoparticle-based aptameric system for the highly selective and sensitive detection of platelet-derived growth factor. Talanta 103:392–397. doi:10.1016/j.talanta.2012.10.087
Zhu Y, Chandra P, Shim YB (2013) Ultrasensitive and selective electrochemical diagnosis of breast cancer based on a hydrazine–Au nanoparticle-aptamer bioconjugate. Anal Chem 85(2):1058–1064. doi:10.1021/ac302923k
Kim JS, Sung JH, Ji JH, Song KS, Lee JH, Kang CS, Yu IJ (2011) In vivo genotoxicity of silver nanoparticles after 90-day silver nanoparticle inhalation exposure. Saf Health Work 2(1):34–38. doi:10.5491/SHAW.2011.2.1.34
Sundaram P, Wower J, Byrne ME (2012) A nanoscale drug delivery carrier using nucleic acid aptamers for extended release of therapeutic. Nanomedicine 8(7):1143–1151. doi:10.1016/j.nano.2012.01.010
Luo YL, Shiao YS, Huang YF (2011) Release of photoactivatable drugs from plasmonic nanoparticles for targeted cancer therapy. ACS Nano 5(10):7796–7804. doi:10.1021/nn201592s
Maeda H (2001) The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul 41:189–207
Etame AB, Smith CA, Chan WC, Rutka JT (2011) Design and potential application of PEGylated gold nanoparticles with size-dependent permeation through brain microvasculature. Nanomedicine 7(6):992–1000. doi:10.1016/j.nano.2011.04.004
Arvizo RR, Rana S, Miranda OR, Bhattacharya R, Rotello VM, Mukherjee P (2011) Mechanism of anti-angiogenic property of gold nanoparticles: role of nanoparticle size and surface charge. Nanomedicine 7(5):580–587. doi:10.1016/j.nano.2011.01.011
Gurunathan S, Lee KJ, Kalishwaralal K, Sheikpranbabu S, Vaidyanathan R, Eom SH (2009) Antiangiogenic properties of silver nanoparticles. Biomaterials 30(31):6341–6350. doi:10.1016/j.biomaterials.2009.08.008
Mukherjee P, Bhattacharya R, Wang P, Wang L, Basu S, Nagy JA, Atala A, Mukhopadhyay D, Soker S (2005) Antiangiogenic properties of gold nanoparticles. Clin Cancer Res 11(9):3530–3534. doi:10.1158/1078-0432.CCR-04-2482
Brown SD, Nativo P, Smith JA, Stirling D, Edwards PR, Venugopal B, Flint DJ, Plumb JA, Graham D, Wheate NJ (2010) Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. J Am Chem Soc 132(13):4678–4684. doi:10.1021/ja908117a
Kemp MM, Kumar A, Mousa S, Dyskin E, Yalcin M, Ajayan P, Linhardt RJ, Mousa SA (2009) Gold and silver nanoparticles conjugated with heparin derivative possess anti-angiogenesis properties. Nanotechnology 20(45):455104. doi:10.1088/0957-4484/20/45/455104
Zhou C, Chen T, Wu C, Zhu G, Qiu L, Cui C, Hou W, Tan W (2015) Aptamer CaCO3 nanostructures: a facile, pH-responsive, specific platform for targeted anticancer theranostics. Chem Asian J 10(1):166–171. doi:10.1002/asia.201403115
Li C, Chen T, Ocsoy I, Zhu G, Yasun E, You M, Wu C, Zheng J, Song E, Huang CZ, Tan W (2014) Gold-coated FeO nanoroses with five unique functions for cancer cell targeting, imaging and therapy. Adv Funct Mater 24(12):1772–1780. doi:10.1002/adfm.201301659
Van Lehn RC, Ricci M, Silva PH, Andreozzi P, Reguera J, Voitchovsky K, Stellacci F, Alexander-Katz A (2014) Lipid tail protrusions mediate the insertion of nanoparticles into model cell membranes. Nature Commun 5:4482. doi: 10.1038/ncomms5482
Sung JH, Ji JH, Song KS, Lee JH, Choi KH, Lee SH, Yu IJ (2011) Acute inhalation toxicity of silver nanoparticles. Toxicol Ind Health 27(2):149–154. doi:10.1177/0748233710382540
Sung JH, Ji JH, Park JD, Song MY, Song KS, Ryu HR, Yoon JU, Jeon KS, Jeong J, Han BS, Chung YH, Chang HK, Lee JH, Kim DW, Kelman BJ, Yu IJ (2011) Subchronic inhalation toxicity of gold nanoparticles. Part Fibre Toxicol 8:16. doi:10.1186/1743-8977-8-16
Blaser SA, Scheringer M, Macleod M, Hungerbuhler K (2008) Estimation of cumulative aquatic exposure and risk due to silver: contribution of nano-functionalized plastics and textiles. Sci Total Environ 390(2–3):396–409. doi:10.1016/j.scitotenv.2007.10.010
Dreher KL (2004) Health and environmental impact of nanotechnology: toxicological assessment of manufactured nanoparticles. Toxicol Sci 77(1):3–5
Seaton A, Tran L, Aitken R, Donaldson K (2010) Nanoparticles, human health hazard and regulation. J R Soc Interface 7[Suppl 1]:S119–129. doi:10.1098/rsif.2009.0252.focus
Shukla R, Bansal V, Chaudhary M, Basu A, Bhonde RR, Sastry M (2005) Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overview. Langmuir 21(23):10644–10654. doi:10.1021/la0513712
Du L, Suo S, Wang G, Jia H, Liu KJ, Zhao B, Liu Y (2013) Mechanism and cellular kinetic studies of the enhancement of antioxidant activity by using surface-functionalized gold nanoparticles. Chemistry 19(4):1281–1287. doi:10.1002/chem.201203506
Chen J, Wang H, Long W, Shen X, Wu D, Song SS, Sun YM, Liu PX, Fan S, Fan F, Zhang XD (2013) Sex differences in the toxicity of polyethylene glycol-coated gold nanoparticles in mice. Int J Nanomedicine 8:2409–2419. doi:10.2147/IJN.S46376
Khan MS, Vishakante GD, Siddaramaiah H (2013) Gold nanoparticles: a paradigm shift in biomedical applications. Adv Colloid Interface Sci. doi:10.1016/j.cis.2013.06.003
Etame AB, Diaz RJ, O’Reilly MA, Smith CA, Mainprize TG, Hynynen K, Rutka JT (2012) Enhanced delivery of gold nanoparticles with therapeutic potential into the brain using MRI-guided focused ultrasound. Nanomedicine 8(7):1133–1142. doi:10.1016/j.nano.2012.02.003
Liu Z, Wu Y, Guo Z, Liu Y, Shen Y, Zhou P, Lu X (2014) Effects of internalized gold nanoparticles with respect to cytotoxicity and invasion activity in lung cancer cells. PLoS ONE 9(6):e99175. doi:10.1371/journal.pone.0099175
Gonzalez VH, Giuliari GP, Banda RM, Guel DA (2009) Intravitreal injection of pegaptanib sodium for proliferative diabetic retinopathy. Br J Ophthalmol 93(11):1474–1478. doi:10.1136/bjo.2008.155663
Cunningham ET Jr, Adamis AP, Altaweel M, Aiello LP, Bressler NM, D’Amico DJ, Goldbaum M, Guyer DR, Katz B, Patel M, Schwartz SD (2005) A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology 112(10):1747–1757. doi:10.1016/j.ophtha.2005.06.007
Ireson CR, Kelland LR (2006) Discovery and development of anticancer aptamers. Mol Cancer Ther 5(12):2957–2962. doi:10.1158/1535-7163.MCT-06-0172
Guarneri V, Dieci MV, Conte P (2012) Enhancing intracellular taxane delivery: current role and perspectives of nanoparticle albumin-bound paclitaxel in the treatment of advanced breast cancer. Expert Opin Pharmacother 13(3):395–406. doi:10.1517/14656566.2012.651127
Hamaguchi T, Matsumura Y, Suzuki M, Shimizu K, Goda R, Nakamura I, Nakatomi I, Yokoyama M, Kataoka K, Kakizoe T (2005) NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br J Cancer 92(7):1240–1246. doi:10.1038/sj.bjc.6602479
Libutti SK, Paciotti GF, Byrnes AA, Alexander HR Jr, Gannon WE, Walker M, Seidel GD, Yuldasheva N, Tamarkin L (2010) Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin Cancer Res 16(24):6139–6149. doi:10.1158/1078-0432.CCR-10-0978
Ramanathan R, Hamburg SI, Borad M, Seetharam M, Kundranda M, Lee P, Frelund P, Gibert M, Mast C, Semple S, Judge A, Crowell A, Vocila L, MacLachlan I, Northfeld DW (2013) A phase I dose escalation study of TKM-080301, a RNA therapeutic directed aptamer against PLK1, in patients with advanced solid tumors Paper presented at the 104th Annual Meeting of the American Association of Cancer Research., Washington, D.C. J Clin Oncol 31, (suppl; abstr TPS2621)
Zuckerman JE, Gritli I, Tolcher A, Heidel JD, Lim D, Morgan R, Chmielowski B, Ribas A, Davis ME, Yen Y (2014) Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc Natl Acad Sci USA 111(31):11449–11454. doi:10.1073/pnas.1411393111
Pottier A, Borghi E, Levy L (2014) New use of metals as nanosized radioenhancers. Anticancer Res 34(1):443–453
Maggiorella L, Barouch G, Devaux C, Pottier A, Deutsch E, Bourhis J, Borghi E, Levy L (2012) Nanoscale radiotherapy with hafnium oxide nanoparticles. Future Oncol 8(9):1167–1181. doi:10.2217/fon.12.96
Jaetao JE, Butler KS, Adolphi NL, Lovato DM, Bryant HC, Rabinowitz I, Winter SS, Tessier TE, Hathaway HJ, Bergemann C, Flynn ER, Larson RS (2009) Enhanced leukemia cell detection using a novel magnetic needle and nanoparticles. Cancer Res 69(21):8310–8316. doi:10.1158/0008-5472.CAN-09-1083
Perche F, Torchilin VP (2013) Recent trends in multifunctional liposomal nanocarriers for enhanced tumor targeting. J Drug Del 2013:705265. doi:10.1155/2013/705265
Dhar S, Kolishetti N, Lippard SJ, Farokhzad OC (2011) Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proc Natl Acad Sci USA 108(5):1850–1855. doi:10.1073/pnas.1011379108
Zhang K, Sefah K, Tang L, Zhao Z, Zhu G, Ye M, Sun W, Goodison S, Tan W (2012) A novel aptamer developed for breast cancer cell internalization. ChemMedChem 7(1):79–84. doi:10.1002/cmdc.201100457
He X, Hai L, Su J, Wang K, Wu X (2011) One-pot synthesis of sustained-released doxorubicin silica nanoparticles for aptamer targeted delivery to tumor cells. Nanoscale 3(7):2936–2942. doi:10.1039/c0nr00913j
Li LL, Xie M, Wang J, Li X, Wang C, Yuan Q, Pang DW, Lu Y, Tan W (2013) A vitamin-responsive mesoporous nanocarrier with DNA aptamer-mediated cell targeting. Chem Commun (Camb) 49(52):5823–5825. doi:10.1039/c3cc41072b
Jalalian SH, Taghdisi SM, Shahidi Hamedani N, Kalat SA, Lavaee P, Zandkarimi M, Ghows N, Jaafari MR, Naghibi S, Danesh NM, Ramezani M, Abnous K (2013) Epirubicin loaded super paramagnetic iron oxide nanoparticle-aptamer bioconjugate for combined colon cancer therapy and imaging in vivo. Eur J Pharm Sci 50(2):191–197. doi:10.1016/j.ejps.2013.06.015
Yu C, Hu Y, Duan J, Yuan W, Wang C, Xu H, Yang XD (2011) Novel aptamer-nanoparticle bioconjugates enhances delivery of anticancer drug to MUC1-positive cancer cells in vitro. PLoS ONE 6(9):e24077. doi:10.1371/journal.pone.0024077
Guo J, Gao X, Su L, Xia H, Gu G, Pang Z, Jiang X, Yao L, Chen J, Chen H (2011) Aptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials 32(31):8010–8020. doi:10.1016/j.biomaterials.2011.07.004
Aravind A, Jeyamohan P, Nair R, Veeranarayanan S, Nagaoka Y, Yoshida Y, Maekawa T, Kumar DS (2012) AS1411 aptamer tagged PLGA-lecithin-PEG nanoparticles for tumor cell targeting and drug delivery. Biotechnol Bioeng 109(11):2920–2931. doi:10.1002/bit.24558
Zhang Z, Balogh D, Wang F, Sung SY, Nechushtai R, Willner I (2013) Biocatalytic release of an anticancer drug from nucleic-acids-capped mesoporous SiO2 Using DNA or molecular biomarkers as triggering stimuli. ACS Nano 7(10):8455–8468. doi:10.1021/nn403772j
Zhou W, Zhou Y, Wu J, Liu Z, Zhao H, Liu J, Ding J (2014) Aptamer-nanoparticle bioconjugates enhance intracellular delivery of vinorelbine to breast cancer cells. J Drug Target 22(1):57–66. doi:10.3109/1061186X.2013.839683
Lin Z, Ma Q, Fei X, Zhang H, Su X (2014) A novel aptamer functionalized CuInS2 quantum dots probe for daunorubicin sensing and near infrared imaging of prostate cancer cells. Anal Chim Acta 818:54–60. doi:10.1016/j.aca.2014.01.057
Li L, Hou J, Liu X, Guo Y, Wu Y, Zhang L, Yang Z (2014) Nucleolin-targeting liposomes guided by aptamer AS1411 for the delivery of siRNA for the treatment of malignant melanomas. Biomaterials 35(12):3840–3850. doi:10.1016/j.biomaterials.2014.01.019
Sayari E, Dinarvand M, Amini M, Azhdarzadeh M, Mollarazi E, Ghasemi Z, Atyabi F (2014) MUC1 aptamer conjugated to chitosan nanoparticles, an efficient targeted carrier designed for anticancer SN38 delivery. Int J Pharm 473(1–2):304–315. doi:10.1016/j.ijpharm.2014.05.041
Shiao YS, Chiu HH, Wu PH, Huang YF (2014) Aptamer-functionalized gold nanoparticles as photoresponsive nanoplatform for Co-drug delivery. ACS Appl Mater Interfaces 6(24):21832–21841. doi:10.1021/am5026243
Kolishetti N, Dhar S, Valencia PM, Lin LQ, Karnik R, Lippard SJ, Langer R, Farokhzad OC (2010) Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proc Natl Acad Sci USA 107(42):17939–17944. doi:10.1073/pnas.1011368107
Kim E, Jung Y, Choi H, Yang J, Suh JS, Huh YM, Kim K, Haam S (2010) Prostate cancer cell death produced by the co-delivery of Bcl-xL shRNA and doxorubicin using an aptamer-conjugated polyplex. Biomaterials 31(16):4592–4599. doi:10.1016/j.biomaterials.2010.02.030
Xiao Z, Farokhzad OC (2012) Aptamer-functionalized nanoparticles for medical applications: challenges and opportunities. ACS Nano 6(5):3670–3676. doi:10.1021/nn301869z
Prasad P, Cheng J, Shuhendler A, Rauth AM, Wu XY (2012) A novel nanoparticle formulation overcomes multiple types of membrane efflux pumps in human breast cancer cells. Drug Deliv Transl Res 2(2):95–105. doi:10.1007/s13346-011-0051-1
Liu M, Chen D, Wang C, Chen X, Wen Z, Cao Y, He H (2015) Intracellular target delivery of 10-hydroxycamptothecin with solid lipid nanoparticles against multidrug resistance. J Drug Target. 13:1–6. doi:10.3109/1061186X.2015.1020427
Qiu L, Chen T, Ocsoy I, Yasun E, Wu C, Zhu G, You M, Han D, Jiang J, Yu R, Tan W (2015) A cell-targeted, size-photocontrollable, nuclear-uptake nanodrug delivery system for drug-resistant cancer therapy. Nano Lett 15(1):457–463. doi:10.1021/nl503777s
Verma A, Stellacci F (2010) Effect of surface properties on nanoparticle-cell interactions. Small 6(1):12–21. doi:10.1002/smll.200901158
marketsandmarkets.com (2013) Aptamers market—technology trend analysis by applications—therapeutics, diagnostics, biosensors, drug discovery, biomarker discovery, research applications with market landscape analysis—global forecasts to 2018.
Chen C, Zhou L, Geng J, Ren J, Qu X (2013) Photosensitizer-incorporated quadruplex DNA-gated nanovechicles for light-triggered, targeted dual drug delivery to cancer cells. Small 9(16):2793–2800, 2653. doi:10.1002/smll.201201916
Hattori Y, Maitani Y (2005) Folate-linked lipid-based nanoparticle for targeted gene delivery. Curr Drug Deliv 2(3):243–252
Pan X, Lee RJ (2004) Tumour-selective drug delivery via folate receptor-targeted liposomes. Expert Opin Drug Deliv 1(1):7–17. doi:10.1517/17425247.1.1.7
Zhao X, Li H, Lee RJ (2008) Targeted drug delivery via folate receptors. Expert Opin Drug Deliv 5(3):309–319. doi:10.1517/17425247.5.3.309
Zuber G, Muller CD, Behr JP (2005) Targeted gene delivery to cancer cells with nanometric DNA particles enveloped with folic acid using a polymerisable anchor. Technol Cancer Res Treat 4(6):637–643
Huang Z, Li H, Huang Q, Chen D, Han J, Wang L, Pan C, Chen W, House MG, Nephew KP, Guo Z (2014) SERPINB2 down-regulation contributes to chemoresistance in head and neck cancer. Mol Carcinog 53(10):777–786. doi:10.1002/mc.22033
Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, Welin S, Schade-Brittinger C, Lacroix A, Jarzab B, Sorbye H, Torpy DJ, Stepan V, Schteingart DE, Arlt W, Kroiss M, Leboulleux S, Sperone P, Sundin A, Hermsen I, Hahner S, Willenberg HS, Tabarin A, Quinkler M, de la Fouchardiere C, Schlumberger M, Mantero F, Weismann D, Beuschlein F, Gelderblom H, Wilmink H, Sender M, Edgerly M, Kenn W, Fojo T, Muller HH, Skogseid B, Group F-AS (2012) Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med 366(23):2189–2197. doi:10.1056/NEJMoa1200966
von Mehren M, Bookman M, Meropol NJ, Weiner LM, Sherman E, Li J, Knoblauch R, Parekh T, Cohen RB (2015) Phase I study of the safety and pharmacokinetics of trabectedin with docetaxel in patients with advanced malignancies. Cancer Chemother Pharmacol 5(5):1047–1055. doi:10.1007/s00280-015-2705-z
Liao L, Liu J, Dreaden EC, Morton SW, Shopsowitz KE, Hammond PT, Johnson JA (2014) A convergent synthetic platform for single-nanoparticle combination cancer therapy: ratiometric loading and controlled release of cisplatin, doxorubicin, and camptothecin. J Am Chem Soc 136(16):5896–5899. doi:10.1021/ja502011g
Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AK, Han MS, Mirkin CA (2006) Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science 312(5776):1027–1030. doi:10.1126/science.1125559
Young KL, Scott AW, Hao L, Mirkin SE, Liu G, Mirkin CA (2012) Hollow spherical nucleic acids for intracellular gene regulation based upon biocompatible silica shells. Nano Lett 12(7):3867–3871. doi:10.1021/nl3020846
Giljohann DA, Seferos DS, Prigodich AE, Patel PC, Mirkin CA (2009) Gene regulation with polyvalent siRNA-nanoparticle conjugates. J Am Chem Soc 131(6):2072–2073. doi:10.1021/ja808719p
Li J, Zheng C, Cansiz S, Wu C, Xu J, Cui C, Liu Y, Hou W, Wang Y, Zhang L, Teng IT, Yang HH, Tan W (2015) Self-assembly of DNA nanohydrogels with controllable size and stimuli-responsive property for targeted gene regulation therapy. J Am Chem Soc 137(4):1412–1415. doi:10.1021/ja512293f
Peterson AM, Heemstra JM (2015) Controlling self-assembly of DNA-polymer conjugates for applications in imaging and drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol 7(3):282-297. doi:10.1002/wnan.1309
Acknowledgments
We gratefully acknowledge support to C.R. from the Charlotte Research Institute, the Center for Biomedical Engineering and Sciences (University of North Carolina at Charlotte), and a Faculty Research grant (University of North Carolina at Charlotte).
Potential Conflict of Interest
CR is funded through NIH/NCI and has grant pending with NIH/NCI and a patent pending. GB has a patent pending. CV has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Benedetto, G., Vestal, C.G. & Richardson, C. Aptamer-Functionalized Nanoparticles as “Smart Bombs”: The Unrealized Potential for Personalized Medicine and Targeted Cancer Treatment. Targ Oncol 10, 467–485 (2015). https://doi.org/10.1007/s11523-015-0371-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-015-0371-z