Abstract
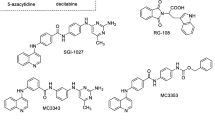
After extensive research on radiochemotherapy, 5-year survival rates of children with high risk neuroblastoma still do not exceed 50%, owing to adverse side-effects exemplified by doxorubicin-induced cardiomyopathy. A promising new approach is the combination of conventional therapies with specific modulation of cell signaling pathways promoting therapeutic resistance, such as inhibition of aberrant kinase activity or re-expression of silenced tumor suppressor genes by means of chromatin remodeling. In this regard, we established a system that allows to identify potential drug targets as well as to validate respective candidate inhibitors in high-risk neuroblastoma model cell lines. Cell culture, drug exposure, shRNA-mediated knockdown and phenotype analysis are integrated into an efficient and versatile single well-based protocol. By utilizing this system, we assessed RG108, SGI-1027 and nanaomycin A, three novel DNA methyltransferase inhibitors that have not been tested in neuroblastoma cell lines so far, for their potential of synergistic anti-tumor activity in combination with doxorubicin. We found that, similarly to azacytidine, SGI-1027 and nanaomycin A mediate synergistic growth inhibition with doxorubicin independently of N-Myc status. However, they display high cytotoxicity but lack global DNA demethylation activity. Secondly, we conducted a lentiviral shRNA screen of F-box proteins, key regulators of protein stability, and identified Fbxw11/β-TrCP2 as well as Fbxo5/Emi1 as potential therapeutic targets in neuroblastoma. These results complement existing studies and underline the reliability and versatility of our single well-based protocol.





Similar content being viewed by others
References
Maris J (2010) Recent advances in neuroblastoma. N Engl J Med 362(23):2202–11
Charlet J, Schnekenburger M, Brown KW, Diederich M (2012) DNA demethylation increases sensitivity of neuroblastoma cells to chemotherapeutic drugs. Biochem Pharmacol 83(7):858–65
Chen ZC, Chen LJ, Cheng JT (2013) Doxorubicin-induced cardiac toxicity is mediated by lowering of peroxisome proliferator-activated receptor δ expression in rats. PPAR Res 2013:456042
Takemura G, Fujiwara H (2007) Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis 49(5):330–52
Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N et al (2006) Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 355(23):2408–17
Cheung NK, Dyer MA (2013) Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat Rev Cancer 13(6):397–411
Esteller M (2008) Epigenetics in cancer. N Engl J Med 358(11):1148–59
Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A et al (2009) Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 10(3):223–32
George RE, Lahti JM, Adamson PC, Zhu K, Finkelstein D, Ingle AM et al (2010) Phase I study of decitabine with doxorubicin and cyclophosphamide in children with neuroblastoma and other solid tumors: a Children's Oncology Group study. Pediatr Blood Cancer 55(4):629–38
Brueckner B, Garcia Boy R, Siedlecki P, Musch T, Kliem HC, Zielenkiewicz P et al (2005) Epigenetic reactivation of tumor suppressor genes by a novel small-molecule inhibitor of human DNA methyltransferases. Cancer Res 65(14):6305–11
Datta J, Ghoshal K, Denny WA, Gamage SA, Brooke DG, Phiasivongsa P et al (2009) A new class of quinoline-based DNA hypomethylating agents reactivates tumor suppressor genes by blocking DNA methyltransferase 1 activity and inducing its degradation. Cancer Res 69(10):4277–85
Kuck D, Caulfield T, Lyko F, Medina-Franco JL (2010) Nanaomycin A selectively inhibits DNMT3B and reactivates silenced tumor suppressor genes in human cancer cells. Mol Cancer Ther 9(11):3015–23
Wiebusch L, Hagemeier C (2010) p53- and p21-dependent premature APC/C-Cdh1 activation in G2 is part of the long-term response to genotoxic stress. Oncogene 29(24):3477–89
Schiesser S, Pfaffeneder T, Sadeghian K, Hackner B, Steigenberger B, Schröder AS et al (2013) Deamination, oxidation, and C-C bond cleavage reactivity of 5-hydroxymethylcytosine, 5-formylcytosine, and 5-carboxycytosine. J Am Chem Soc 135(39):14593–9
Riccardi C, Nicoletti I (2006) Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc 1(3):1458–61
Fulda S, Lutz W, Schwab M, Debatin K (1999) MycN sensitizes neuroblastoma cells for drug-induced apoptosis. Oncogene 18(7):1479–86
Goldschneider D, Horvilleur E, Plassa LF, Guillaud-Bataille M, Million K, Wittmer-Dupret E et al (2006) Expression of C-terminal deleted p53 isoforms in neuroblastoma. Nucleic Acids Res 34(19):5603–12
Spänkuch-Schmitt B, Bereiter-Hahn J, Kaufmann M, Strebhardt K (2002) Effect of RNA silencing of polo-like kinase-1 (PLK1) on apoptosis and spindle formation in human cancer cells. J Natl Cancer Inst 94(24):1863–77
Spänkuch B, Heim S, Kurunci-Csacsko E, Lindenau C, Yuan J, Kaufmann M et al (2006) Down-regulation of Polo-like kinase 1 elevates drug sensitivity of breast cancer cells in vitro and in vivo. Cancer Res 66(11):5836–46
Maier B, Wendt S, Vanselow J, Wallach T, Reischl S, Oehmke S et al (2009) A large-scale functional RNAi screen reveals a role for CK2 in the mammalian circadian clock. Genes Dev 23(6):708–18
Machida YJ, Dutta A (2007) The APC/C inhibitor, Emi1, is essential for prevention of rereplication. Genes Dev 21(2):184–94
Shimizu N, Nakajima NI, Tsunematsu T, Ogawa I, Kawai H, Hirayama R, et al (2013)Selective enhancing effect of early mitotic inhibitor 1 depletion on the sensitivity of doxorubicin or X-ray treatment in human cancer cells. J Biol Chem. May
Tang W, Li Y, Yu D, Thomas-Tikhonenko A, Spiegelman VS, Fuchs SY (2005) Targeting beta-transducin repeat-containing protein E3 ubiquitin ligase augments the effects of antitumor drugs on breast cancer cells. Cancer Res 65(5):1904–8
Hegde V, McFarlane RJ, Taylor EM, Price C (1996) The genetics of the repair of 5-azacytidine-mediated DNA damage in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet 251(4):483–92
García-Domínguez P, Dell'aversana C, Alvarez R, Altucci L, de Lera AR (2013) Synthetic approaches to DNMT inhibitor SGI-1027 and effects on the U937 leukemia cell line. Bioorg Med Chem Lett 23(6):1631–5
Hagemann S, Kuck D, Stresemann, Carlo, Prinz F, Brueckner B, et al (2012) Antiproliferative effects of DNA methyltransferase 3B depletion are not associated with DNA demethylation. PLoS ONE; p. e36125.
Qiu YY, Mirkin BL, Dwivedi RS (2005) Inhibition of DNA methyltransferase reverses cisplatin induced drug resistance in murine neuroblastoma cells. Cancer Detect Prev 29(5):456–63
Qiu YY, Mirkin BL, Dwivedi RS (2002) Differential expression of DNA-methyltransferases in drug resistant murine neuroblastoma cells. Cancer Detect Prev 26(6):444–53
Schlabach MR, Luo J, Solimini NL, Hu G, Xu Q, Li MZ et al (2008) Cancer proliferation gene discovery through functional genomics. Science 319(5863):620–4
Gluschnaider U, Hidas G, Cojocaru G, Yutkin V, Ben-Neriah Y, Pikarsky E (2010) beta-TrCP inhibition reduces prostate cancer cell growth via upregulation of the aryl hydrocarbon receptor. PLoS One 5(2):e9060
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
Effects of azacytidine, RG108, SGI-1027 and nanaomycin A +/-doxorubicin on cell cycle distribution. Cell cycle distribution measured by flow cytometry using propidium iodide fluorescence labeling: DNA content of individual cells is represented by pulse area (PE-A). Pulse width (PE-W) was used to exclude cell doublets. a) SK-N-AS and SH-EP cells (N-Myc single copy).b) LAN-1 and SK-N-BE(2) cells (N-Myc amplified). (PDF 78 kb)
Online Resource 2
Effects of azacytidine, RG108, SGI-1027 and nanaomycin A +/-doxorubicin on apoptosis quantified with flow cytometry using Annexin V (AV) and propidium iodide (PI) fluorescence labeling. Viable and dead cells are labeled AV-/PI- and AV+/PI+, while early and late apoptosis are characterized as AV+/PI- and AV+/PI+. a) SK-N-AS and SH-EP cells (N-Myc single copy). b) LAN-1 and SK-N-BE(2) cells (N-Myc amplified). (PDF 101 kb)
Online Resource 3
Overview of shRNAs against F-box proteins employed in the paper (PDF 14 kb)
Rights and permissions
About this article
Cite this article
Penter, L., Maier, B., Frede, U. et al. A rapid screening system evaluates novel inhibitors of DNA methylation and suggests F-box proteins as potential therapeutic targets for high-risk neuroblastoma. Targ Oncol 10, 523–533 (2015). https://doi.org/10.1007/s11523-014-0354-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-014-0354-5