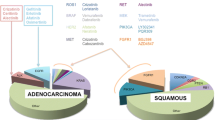
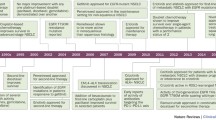
Abstract
Chemotherapy represents the mainstay of non-small cell lung cancer (NSCLC) treatment, but response is usually observed in only one out of three patients. Massive efforts have been carried out to identify biomarkers that might help clinicians to choose appropriate drugs, by identifying potentially sensitive subjects and spare toxicities in patients who are unlikely to benefit from treatment. Low excision repair cross-complementation group 1 (ERCC1) and ribonucleotide reductase M1 (RRM1) levels have been associated with increased sensitivity to cisplatin and gemcitabine, respectively, while reduced class III β-tubulin expression has been associated with taxane activity. Initial prospective studies showed the feasibility of a customized approach based on biomarker assessment, and phase III trials will hopefully provide further validation of this approach. The impact of biomarkers for patient selection has now been well established for tyrosine kinase inhibitors (TKIs) of the epidermal growth factor receptor (EGFR), with EGFR mutations emerging as the most reliable predictor for improved outcome. Relevant clinical issues are represented by the identification of patients who can be reasonably excluded from treatment and by the development of therapeutic approaches able to overcome acquired resistance to anti-EGFR strategies.
Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer statistics, 2009. CA Cancer J Clin 59(4):225–249
Schiller JH, Harrington D, Belani CP et al (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346(2):92–98
Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J (2004) Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 350(4):351–360
Winton T, Livingston R, Johnson D et al (2005) Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 352(25):2589–2597
Douillard JY, Rosell R, De Lena M et al (2006) Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (adjuvant navelbine international trialist association [anita]): a randomised controlled trial. Lancet Oncol 7(9):719–727
Fuertesa MA, Castillab J, Alonsoa C, Perez JM (2003) Cisplatin biochemical mechanism of action: from cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr Med Chem 10(3):257–266
De Las Penas R, Sanchez-Ronco M, Alberola V et al (2006) Polymorphisms in DNA repair genes modulate survival in cisplatin/gemcitabine-treated non-small-cell lung cancer patients. Ann Oncol 17(4):668–675
Ceppi P, Volante M, Novello S et al (2006) ERCC1 and RRM1 gene expressions but not EGFR are predictive of shorter survival in advanced non-small-cell lung cancer treated with cisplatin and gemcitabine. Ann Oncol 17(12):1818–1825
Lord RV, Brabender J, Gandara D et al (2002) Low ERCC1 expression correlates with prolonged survival after cisplatin plus gemcitabine chemotherapy in non-small cell lung cancer. Clin Cancer Res 8(7):2286–2291
Rosell R, Scagliotti G, Danenberg KD et al (2003) Transcripts in pretreatment biopsies from a three-arm randomized trial in metastatic non-small-cell lung cancer. Oncogene 22(23):3548–3553
Tibaldi C, Giovannetti E, Vasile E et al (2008) Correlation of CDA, ERCC1, and XPD polymorphisms with response and survival in gemcitabine/cisplatin-treated advanced non-small cell lung cancer patients. Clin Cancer Res 14(6):1797–1803
Wang G, Dombkowski A, Chuang L, Xu XX (2004) The involvement of XPC protein in the cisplatin DNA damaging treatment-mediated cellular response. Cell Res 14(4):303–314
Li J, Li ZN, Du YJ, Li XQ, Bao QL, Chen P (2009) Expression of MRP1, BCRP, LRP, and ERCC1 in advanced non-small-cell lung cancer: correlation with response to chemotherapy and survival. Clin Lung Cancer 10(6):414–421
Booton R, Ward T, Ashcroft L, Morris J, Heighway J, Thatcher N (2007) ERCC1 mRNA expression is not associated with response and survival after platinum-based chemotherapy regimens in advanced non-small cell lung cancer. J Thorac Oncol 2(10):902–906
Olaussen KA, Dunant A, Fouret P et al (2006) DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med 355(10):983–991
Holm B, Mellemgaard A, Skov T, Skov BG (2009) Different impact of excision repair cross-complementation group 1 on survival in male and female patients with inoperable non-small-cell lung cancer treated with carboplatin and gemcitabine. J Clin Oncol 27(26):4254–4259
Reynolds C, Obasaju C, Schell MJ et al (2009) Randomized phase III trial of gemcitabine-based chemotherapy with in situ RRM1 and ERCC1 protein levels for response prediction in non-small-cell lung cancer. J Clin Oncol 27(34):5808–5815
Ryu JS, Hong YC, Han HS et al (2004) Association between polymorphisms of ERCC1 and XPD and survival in non-small-cell lung cancer patients treated with cisplatin combination chemotherapy. Lung Cancer 44(3):311–316
Isla D, Sarries C, Rosell R et al (2004) Single nucleotide polymorphisms and outcome in docetaxel-cisplatin-treated advanced non-small-cell lung cancer. Ann Oncol 15(8):1194–1203
Kalikaki A, Kanaki M, Vassalou H et al (2009) DNA repair gene polymorphisms predict favorable clinical outcome in advanced non-small-cell lung cancer. Clin Lung cancer 10(2):118–123
Takenaka T, Yano T, Kiyohara C et al (2009) Effects of excision repair cross-complementation group 1 (ERCC1) single nucleotide polymorphisms on the prognosis of non-small cell lung cancer patients. Lung Cancer (Amsterdam, Netherlands) 67(1):101–107
Mullan PB, Quinn JE, Gilmore PM et al (2001) BRCA1 and GADD45 mediated G2/M cell cycle arrest in response to antimicrotubule agents. Oncogene 20(43):6123–6131
Kennedy RD, Quinn JE, Johnston PT, Harkin DP (2002) BRCA1: mechanisms of inactivation and implications for management of patients. Lancet 360(9338):1007–1014
Husain A, He G, Venkatraman ES, Spriggs DR (1998) BRCA1 up-regulation is associated with repair-mediated resistance to cis-diamminedichloroplatinum(II). Cancer Res 58(6):1120–1123
Abbott DW, Thompson ME, Robinson-Benion C, Tomlinson G, Jensen RA, Holt JT (1999) BRCA1 expression restores radiation resistance in BRCA1-defective cancer cells through enhancement of transcription-coupled DNA repair. J Bio Chem 274(26):18808–18812
Lafarge S, Sylvain V, Ferrara M, Bignon YJ (2001) Inhibition of BRCA1 leads to increased chemoresistance to microtubule-interfering agents, an effect that involves the JNK pathway. Oncogene 20(45):6597–6606
Taron M, Rosell R, Felip E et al (2004) BRCA1 mRNA expression levels as an indicator of chemoresistance in lung cancer. Hum Mol Genet 13(20):2443–2449
Boukovinas I, Papadaki C, Mendez P et al (2008) Tumor BRCA1, RRM1 and RRM2 mRNA expression levels and clinical response to first-line gemcitabine plus docetaxel in non-small-cell lung cancer patients. PloS one 3(11):e3695
Kim HT, Lee JE, Shin ES et al (2008) Effect of BRCA1 haplotype on survival of non-small-cell lung cancer patients treated with platinum-based chemotherapy. J Clin Oncol 26(36):5972–5979
Mackey JR, Mani RS, Selner M et al (1998) Functional nucleoside transporters are required for gemcitabine influx and manifestation of toxicity in cancer cell lines. Cancer Res 58(19):4349–4357
Plunkett W, Huang P, Xu YZ, Heinemann V, Grunewald R, Gandhi V (1995) Gemcitabine: metabolism, mechanisms of action, and self-potentiation. Semin Oncol 22(4 Suppl 11):3–10
Achiwa H, Oguri T, Sato S, Maeda H, Niimi T, Ueda R (2004) Determinants of sensitivity and resistance to gemcitabine: the roles of human equilibrative nucleoside transporter 1 and deoxycytidine kinase in non-small cell lung cancer. Cancer Sci 95(9):753–757
Sebastiani V, Ricci F, Rubio-Viqueira B et al (2006) Immunohistochemical and genetic evaluation of deoxycytidine kinase in pancreatic cancer: relationship to molecular mechanisms of gemcitabine resistance and survival. Clin Cancer Res 12(8):2492–2497
Lamba JK, Crews K, Pounds S et al (2007) Pharmacogenetics of deoxycytidine kinase: identification and characterization of novel genetic variants. J Pharmacol Exp Ther 323(3):935–945
Al-Madhoun AS, Van Der Wilt CL, Loves WJ et al (2004) Detection of an alternatively spliced form of deoxycytidine kinase mRNA in the 2′-2′-difluorodeoxycytidine (gemcitabine)-resistant human ovarian cancer cell line ag6000. Biochem Pharm 68(4):601–609
Seve P, Mackey JR, Isaac S et al (2005) cN-II expression predicts survival in patients receiving gemcitabine for advanced non-small cell lung cancer. Lung Cancer (Amsterdam, Netherlands) 49(3):363–370
Bengala C, Guarneri V, Giovannetti E et al (2005) Prolonged fixed dose rate infusion of gemcitabine with autologous haemopoietic support in advanced pancreatic adenocarcinoma. Br J Cancer 93(1):35–40
Giovannetti E, Del Tacca M, Mey V et al (2006) Transcription analysis of human equilibrative nucleoside transporter-1 predicts survival in pancreas cancer patients treated with gemcitabine. Cancer Res 66(7):3928–3935
Van Haperen VW, Veerman G, Vermorken JB, Pinedo HM, Peters G (1996) Regulation of phosphorylation of deoxycytidine and 2′, 2′-difluorodeoxycytidine (gemcitabine); effects of cytidine 5′-triphosphate and uridine 5′-triphosphate in relation to chemosensitivity for 2′, 2′-difluorodeoxycytidine. Biochem Pharmacol 51(7):911–918
Yonemori K, Ueno H, Okusaka T et al (2005) Severe drug toxicity associated with a single-nucleotide polymorphism of the cytidine deaminase gene in a Japanese cancer patient treated with gemcitabine plus cisplatin. Clin Cancer Res 11(7):2620–2624
Sugiyama E, Kaniwa N, Kim SR et al (2007) Pharmacokinetics of gemcitabine in Japanese cancer patients: the impact of a cytidine deaminase polymorphism. J Clin Oncol 25(1):32–42
Rosell R, Felip E, Taron M et al (2004) Gene expression as a predictive marker of outcome in stage IIB-IIIA-IIIB non-small cell lung cancer after induction gemcitabine-based chemotherapy followed by resectional surgery. Clin Cancer Res 10(12 Pt 2):4215s–4219s
Rosell R, Danenberg KD, Alberola V et al (2004) Ribonucleotide reductase messenger RNA expression and survival in gemcitabine/cisplatin-treated advanced non-small cell lung cancer patients. Clin Cancer Res 10(4):1318–1325
Souglakos J, Boukovinas I, Taron M et al (2008) Ribonucleotide reductase subunits M1 and M2 mRNA expression levels and clinical outcome of lung adenocarcinoma patients treated with docetaxel/gemcitabine. Br J Cancer 98(10):1710–1715
Zheng Z, Chen T, Li X, Haura E, Sharma A, Bepler G (2007) DNA synthesis and repair genes RRM1 and ERCC1 in lung cancer. N Engl J Med 356(8):800–808
Bepler G, Zheng Z, Gautam A et al (2005) Ribonucleotide reductase M1 gene promoter activity, polymorphisms, population frequencies, and clinical relevance. Lung Cancer (Amsterdam, Netherlands) 47(2):183–192
Kwon WS, Rha SY, Choi YH et al (2006) Ribonucleotide reductase M1 (RRM1) 2464g>a polymorphism shows an association with gemcitabine chemosensitivity in cancer cell lines. Pharmacogenetics Genom 16(6):429–438
Musacchio A, Salmon ED (2007) The spindle-assembly checkpoint in space and time. Nat Rev 8(5):379–393
Kavallaris M, Burkhart CA, Horwitz SB (1999) Antisense oligonucleotides to class III beta-tubulin sensitize drug-resistant cells to taxol. Br J Cancer 80(7):1020–1025
Hari M, Yang H, Zeng C, Canizales M, Cabral F (2003) Expression of class III beta-tubulin reduces microtubule assembly and confers resistance to paclitaxel. Cell Motil Cytoskeleton 56(1):45–56
Kamath K, Wilson L, Cabral F, Jordan MA (2005) BetaIII-tubulin induces paclitaxel resistance in association with reduced effects on microtubule dynamic instability. J Biol Chem 280(13):12902–12907
Seve P, Mackey J, Isaac S et al (2005) Class III beta-tubulin expression in tumor cells predicts response and outcome in patients with non-small cell lung cancer receiving paclitaxel. Mol Cancer Ther 4(12):2001–2007
Kang CH, Jang BG, Kim DW et al (2009) The prognostic significance of ERCC1, BRCA1, XRCC1, and betaIII-tubulin expression in patients with non-small cell lung cancer treated by platinum- and taxane-based neoadjuvant chemotherapy and surgical resection. Lung Cancer Aug 15
Azuma K, Sasada T, Kawahara A et al (2009) Expression of ERCC1 and class III beta-tubulin in non-small cell lung cancer patients treated with carboplatin and paclitaxel. Lung Cancer (Amsterdam, Netherlands) 64(3):326–333
Hayashi Y, Kuriyama H, Umezu H et al (2009) Class III beta-tubulin expression in tumor cells is correlated with resistance to docetaxel in patients with completely resected non-small-cell lung cancer. Intern Med (Tokyo, Japan) 48(4):203–208
Cobo M, Isla D, Massuti B et al (2007) Customizing cisplatin based on quantitative excision repair cross-complementing 1 mRNA expression: a phase III trial in non-small-cell lung cancer. J Clin Oncol 25(19):2747–2754
Simon G, Sharma A, Li X et al (2007) Feasibility and efficacy of molecular analysis-directed individualized therapy in advanced non-small-cell lung cancer. J Clin Oncol 25(19):2741–2746
Rosell R, Perez-Roca L, Sanchez JJ et al (2009) Customized treatment in non-small-cell lung cancer based on EGFR mutations and BRCA1 mRNA expression. PloS one 4(5):e5133
Arteaga CL (2002) Overview of epidermal growth factor receptor biology and its role as a therapeutic target in human neoplasia. Semin Oncol 29(5 Suppl 14):3–9
Fukuoka M, Yano S, Giaccone G et al (2003) Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (the ideal 1 trial). J Clin Oncol 21(12):2237–2246
Kris MG, Natale RB, Herbst RS et al (2003) Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA 290(16):2149–2158
Perez-Soler R, Chachoua A, Hammond LA et al (2004) Determinants of tumor response and survival with erlotinib in patients with non—small-cell lung cancer. J Clin Oncol 22(16):3238–3247
Gatzemeier U, Pluzanska A, Szczesna A et al (2007) Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the tarceva lung cancer investigation trial. J Clin Oncol 25(12):1545–1552
Giaccone G, Herbst RS, Manegold C et al (2004) Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial—Intact 1. J Clin Oncol 22(5):777–784
Herbst RS, Giaccone G, Schiller JH et al (2004) Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial—Intact 2. J Clin Oncol 22(5):785–794
Herbst RS, Prager D, Hermann R et al (2005) Tribute: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 23(25):5892–5899
Shepherd FA, Rodrigues Pereira J, Ciuleanu T et al (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353(2):123–132
Thatcher N, Chang A, Parikh P et al (2005) Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa survival evaluation in lung cancer). Lancet 366(9496):1527–1537
Kim ES, Hirsh V, Mok T et al (2008) Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (interest): a randomised phase III trial. Lancet 372(9652):1809–1818
Maruyama R, Nishiwaki Y, Tamura T et al (2008) Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol 26(26):4244–4252
Lee D, Kim S, Park K et al (2008) A randomized open-label study of gefitinib versus docetaxel in patients with advanced/metastatic non-small cell lung cancer (NSCLC) who have previously received platinum-based chemotherapy. Proc Am Soc Clin Oncol 26:A8025
Cufer T, Vrdoljak E, Gaafar R, Erensoy I, Pemberton K (2006) Phase II, open-label, randomized study (sign) of single-agent gefitinib (Iressa) or docetaxel as second-line therapy in patients with advanced (stage IIIB or IV) non-small-cell lung cancer. Anticancer Drugs 17(4):401–409
Shepherd FA, Douillard J, Fukuoka M et al (2009) Comparison of gefitinib and docetaxel in patients with pretreated advanced non-small cell lung cancer (NSCLC): meta-analysis from four clinical trials. Proc Am Soc Clin Oncol 27(15S):A8011
Cappuzzo F, Ciuleanu T, Stelmakh L et al (2009) Saturn: a double-blind, randomized, phase III study of maintenance erlotinib versus placebo following nonprogression with first-line platinum-based chemotherapy in patients with advanced NSCLC. Proc Am Soc Clin Oncol 27(15S):A8001
Miller VA, O’Connor P, Soh C, Kabbinavar F (2009) A randomized, double-blind, placebo-controlled, phase IIIb trial (atlas) comparing bevacizumab (b) therapy with or without erlotinib (e) after completion of chemotherapy with b for first-line treatment of locally advanced, recurrent, or metastatic non-small cell lung cancer (NSCLC). Proc Am Soc Clin Oncol 27(18S):LBA002
Cappuzzo F, Coudert BP, Wierzbicki R et al (2009) Efficacy and safety of erlotinib as first-line maintenance in NSCLC following non-progression with chemotherapy: results from the phase III saturn study. Presented at 13th World Conference on Lung Cancer, San Francisco
Hainsworth J, Herbst RS (2008) A phase III, multicenter, placebo-controlled, double-blind, randomized clinical trial to evaluate the efficacy of bevacizumab (Avastin®) in combination with erlotinib (Tarceva®) compared with erlotinib alone for treatment of advanced non-small cell lung cancer after failure of standard first-line chemotherapy (beta). Presented at Chicago Multidisciplinary Symposium in Thoracic Oncology
Kimura H, Fujiwara Y, Sone T et al (2006) EGFR mutation status in tumour-derived DNA from pleural effusion fluid is a practical basis for predicting the response to gefitinib. Br J Cancer 95(10):1390–1395
Lin WC, Chiu CH, Liou JL, Chen YM, Perng RP, Tsai CM (2006) Gefitinib as front-line treatment in chinese patients with advanced non-small-cell lung cancer. Lung Cancer 54(2):193–199
Niho S, Kubota K, Goto K et al (2006) First-line single agent treatment with gefitinib in patients with advanced non-small-cell lung cancer: a phase II study. J Clin Oncol 24(1):64–69
Suzuki R, Hasegawa Y, Baba K et al (2006) A phase II study of single-agent gefitinib as first-line therapy in patients with stage IV non-small-cell lung cancer. Br J Cancer 94(11):1599–1603
Cappuzzo F, Ligorio C, Janne PA et al (2007) Prospective study of gefitinib in epidermal growth factor receptor fluorescence in situ hybridization-positive/phospho-Akt-positive or never smoker patients with advanced non-small-cell lung cancer: the oncobell trial. J Clin Oncol 25(16):2248–2255
Lee DH, Han JY, Yu SY et al (2006) The role of gefitinib treatment for korean never-smokers with advanced or metastatic adenocarcinoma of the lung: a prospective study. J Thorac Oncol 1(9):965–971
Mok TS, Wu YL, Thongprasert S et al (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361(10):947–957
Lee JS, Park K, Kim SW et al (2009) A randomized phase III study of gefitinib versus standard chemotherapy (gemcitabine plus cisplatin) as a first-line treatment for never-smokers with advanced or metastatic adenocarcinoma of the lung. Presented at 13th World Conference on Lung Cancer, San Francisco
Sandler A, Gray R, Perry MC et al (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355(24):2542–2550
Reck M, Von Pawel J, Zatloukal P et al (2009) Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: avail. J Clin Oncol 27(8):1227–1234
Scagliotti GV, Parikh P, Von Pawel J et al (2008) Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 26(21):3543–3551
Lynch TJ, Bell DW, Sordella R et al (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350(21):2129–2139
Paez JG, Janne PA, Lee JC et al (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304(5676):1497–1500
Pao W, Miller V, Zakowski M et al (2004) EGF receptor gene mutations are common in lung cancers from “Never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA 101(36):13306–13311
Pham D, Kris MG, Riely GJ et al (2006) Use of cigarette-smoking history to estimate the likelihood of mutations in epidermal growth factor receptor gene exons 19 and 21 in lung adenocarcinomas. J Clin Oncol 24(11):1700–1704
Cappuzzo F, Hirsch FR, Rossi E et al (2005) Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst 97(9):643–655
Han SW, Kim TY, Hwang PG et al (2005) Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol 23(11):2493–2501
Huang SF, Liu HP, Li LH et al (2004) High frequency of epidermal growth factor receptor mutations with complex patterns in non-small cell lung cancers related to gefitinib responsiveness in Taiwan. Clin Cancer Res 10(24):8195–8203
Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T (2004) Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res 64(24):8919–8923
Marchetti A, Martella C, Felicioni L et al (2005) EGFR mutations in non-small-cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J Clin Oncol 23(4):857–865
Mitsudomi T, Kosaka T, Endoh H et al (2005) Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol 23(11):2513–2520
Shigematsu H, Lin L, Takahashi T et al (2005) Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 97(5):339–346
Tokumo M, Toyooka S, Kiura K et al (2005) The relationship between epidermal growth factor receptor mutations and clinicopathologic features in non-small cell lung cancers. Clin Cancer Res 11(3):1167–1173
Yang SH, Mechanic LE, Yang P et al (2005) Mutations in the tyrosine kinase domain of the epidermal growth factor receptor in non-small cell lung cancer. Clin Cancer Res 11(6):2106–2110
Tsao MS, Sakurada A, Cutz JC et al (2005) Erlotinib in lung cancer—molecular and clinical predictors of outcome. N Engl J Med 353(2):133144
Asahina H, Yamazaki K, Kinoshita I et al (2006) A phase II trial of gefitinib as first-line therapy for advanced non-small cell lung cancer with epidermal growth factor receptor mutations. Br J Cancer 95(8):998–1004
Inoue A, Suzuki T, Fukuhara T et al (2006) Prospective phase II study of gefitinib for chemotherapy-naive patients with advanced non-small-cell lung cancer with epidermal growth factor receptor gene mutations. J Clin Oncol 24(21):3340–3346
Sequist LV, Martins RG, Spigel D et al (2008) First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol 26(15):2442–2449
Sunaga N, Tomizawa Y, Yanagitani N et al (2007) Phase II prospective study of the efficacy of gefitinib for the treatment of stage III/IV non-small cell lung cancer with EGFR mutations, irrespective of previous chemotherapy. Lung Cancer 56(3):383–389
Sutani A, Nagai Y, Udagawa K et al (2006) Gefitinib for non-small-cell lung cancer patients with epidermal growth factor receptor gene mutations screened by peptide nucleic acid-locked nucleic acid PCR clamp. Br J Cancer 95(11):1483–1489
Tamura K, Okamoto I, Kashii T et al (2008) Multicentre prospective phase II trial of gefitinib for advanced non-small cell lung cancer with epidermal growth factor receptor mutations: results of the west Japan thoracic oncology group trial (WJTOG0403). Br J Cancer 98(5):907–914
Yoshida K, Yatabe Y, Park JY et al (2007) Prospective validation for prediction of gefitinib sensitivity by epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer. J Thorac Oncol 2(1):22–28
Sugio K, Uramoto H, Onitsuka T et al (2009) Prospective phase II study of gefitinib in non-small cell lung cancer with epidermal growth factor receptor gene mutations. Lung Cancer 64(3):314–318
Brugger W, Triller N, Blasinska-Morawiec M et al (2009) Biomarker analyses from the phase III placebo-controlled Saturn study of maintenance erlotinib following first-line chemotherapy for advanced NSCLC. Proc Am Soc Clin Oncol 27(15S):A8020
Kubota K, Watanabe K, Kunitoh H et al (2004) Phase III randomized trial of docetaxel plus cisplatin versus vindesine plus cisplatin in patients with stage IV non-small-cell lung cancer: the Japanese taxotere lung cancer study group. J Clin Oncol 22(2):254–261
Takano T, Fukui T, Ohe Y et al (2008) EGFR mutations predict survival benefit from gefitinib in patients with advanced lung adenocarcinoma: a historical comparison of patients treated before and after gefitinib approval in Japan. J Clin Oncol 26(34):5589–5595
Kobayashi K, Inoue A, Maemondo M et al (2009) First-line gefitinib versus first-line chemotherapy by carboplatin (CBDCA) plus paclitaxel (TXL) in non-small cell lung cancer (NSCLC) patients (pts) with EGFR mutations: a phase III study (002) by North East Japan Gefitinib Study Group. Proc Am Soc Clin Oncol 27(15S):A8016
Cappuzzo F, Gregorc V, Rossi E et al (2003) Gefitinib in pretreated non-small-cell lung cancer (NSCLC): analysis of efficacy and correlation with HER2 and epidermal growth factor receptor expression in locally advanced or metastatic NSCLC. J Clin Oncol 21(14):2658–2663
Helfrich BA, Raben D, Varella-Garcia M et al (2006) Antitumor activity of the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor gefitinib (ZD1839, Iressa) in non-small cell lung cancer cell lines correlates with gene copy number and EGFR mutations but not EGFR protein levels. Clin Cancer Res 12(23):7117–7125
Hirsch FR, Varella-Garcia M, Bunn PA Jr et al (2006) Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer. J Clin Oncol 24(31):5034–5042
Hirsch FR, Varella-Garcia M, Cappuzzo F et al (2007) Combination of EGFR gene copy number and protein expression predicts outcome for advanced non-small-cell lung cancer patients treated with gefitinib. Ann Oncol 18(4):752–760
Zhu CQ, Da Cunha Santos G, Ding K et al (2008) Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada clinical trials group study br.21. J Clin Oncol 26(26):4268–4275
Mok TS, To KF, Srimunimimit V et al (2009) Clinical outcomes of patients with epidermal growth factor receptor (EGFR) mutations (mut) in ipass (Iressa™ Pan Asia Study). Presented at 13th World Conference on Lung Cancer, San Francisco
Soulières D, Ciuleanu TE, Stelmakh LV et al (2009) Is there a relationship between increased EGFR gene copy number and the presence of activating EGFR mutations in advanced NSCLC? Presented at 13th World Conference on Lung Cancer, San Francisco
Cappuzzo F, Marchetti A, Skokan M et al (2009) Increased MET gene copy number negatively affects survival of surgically resected non-small cell lung cancer patients. J Clin Oncol 27(10):1667–1674
Eberhard DA, Johnson BE, Amler LC et al (2005) Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol 23(25):5900–5909
Suzuki T, Nakagawa T, Endo H et al (2003) The sensitivity of lung cancer cell lines to the EGFR-selective tyrosine kinase inhibitor ZD1839 (‘Iressa’) is not related to the expression of EGFR or HER-2 or to K-ras gene status. Lung Cancer 42(1):35–41
Massarelli E, Varella-Garcia M, Tang X et al (2007) KRAS mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Clin Cancer Res 13(10):2890–2896
Soda M, Choi YL, Enomoto M et al (2007) Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 448(7153):561–566
Koivunen JP, Mermel C, Zejnullahu K et al (2008) EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res 14(13):4275–4283
Shaw AT, Yeap BY, Mino-Kenudson M et al (2009) Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 27(26):4247–4253
Shimamura T, Ji H, Minami Y et al (2006) Non-small-cell lung cancer and Ba/F3 transformed cells harboring the ERBB2 G776insV_G/C mutation are sensitive to the dual-specific epidermal growth factor receptor and ERBB2 inhibitor HKI-272. Cancer Res 66(13):6487–6491
Stephens P, Hunter C, Bignell G et al (2004) Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature 431(7008):525–526
She QB, Solit D, Basso A, Moasser MM (2003) Resistance to gefitinib in PTEN-null HER-overexpressing tumor cells can be overcome through restoration of PTEN function or pharmacologic modulation of constitutive phosphatidylinositol 3′-kinase/Akt pathway signaling. Clin Cancer Res 9(12):4340–4346
Cappuzzo F, Janne PA, Skokan M et al (2009) MET increased gene copy number and primary resistance to gefitinib therapy in non-small-cell lung cancer patients. Ann Oncol 20(2):298–304
Yano S, Wang W, Li Q et al (2008) Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res 68(22):9479–9487
Pao W, Miller VA, Politi KA et al (2005) Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Medicine 2(3):e73
Yun CH, Mengwasser KE, Toms AV et al (2008) The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci USA 105(6):2070–2075
Engelman JA, Zejnullahu K, Gale CM et al (2007) PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res 67(24):11924–11932
Engelman JA, Zejnullahu K, Mitsudomi T et al (2007) MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316(5827):1039–1043
Bean J, Brennan C, Shih JY et al (2007) MET amplification occurs with or without T790m mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA 104(52):20932–20937
Conflict of Interest statement
No funds were received in support of this study and no benefits in any form have been or will be received from a commercial party related to the subject of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toschi, L., Cappuzzo, F. Impact of biomarkers on non-small cell lung cancer treatment. Targ Oncol 5, 5–17 (2010). https://doi.org/10.1007/s11523-010-0132-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-010-0132-y