Abstract
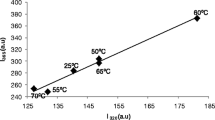
Time-resolved studies were performed for tryptic proteolysis of β-casein in media containing 10–40 % (v/v) ethanol at 37 °C and pH 7.9. The peptide bond demasking, the process which implies the removal of steric obstacles shielding polypeptide sites against enzymatic attack, was quantitatively evaluated with fluorescence spectroscopy by monitoring the exposure of Trp residues to the aqueous polar medium. This process obeys a first-order kinetic law that allowed us the determination of the rate constants of demasking k d . The fraction of initially masked bonds m, being able to convert during proteolysis to the demasked ones, and the fraction of unhydrolysable bonds n were calculated within the framework of a two-step model with consecutive demasking and hydrolysis steps. Parameters m and n were shown to decrease and increase, respectively, with the addition of ethanol.







Similar content being viewed by others
References
M.M. Vorob’ev, V. Vogel, G. Güler, W. Mäntele, Food Biophys. 6, 519–526 (2011)
M.M. Vorob’ev, J. Mol. Catal. B 58, 146–152 (2009)
W.K. Russell, Z.Y. Park, D.H. Russell, Anal. Chem. 73, 2682–2685 (2001)
M.B. Strader, D.L. Tabb, W.J. Hervey, C. Pan, G.B. Hurst, Anal. Chem. 78, 125–134 (2006)
K.G. Welinder, Anal. Biochem. 1974, 54–64 (1988)
B. Tchorbanov, I. Iliev, Enzym. Microb. Technol. 15, 974–978 (1993)
K.M. Clegg, A.D. Mc-Millan, J. Food Technol. 9, 21–29 (1974)
I. Svendsen, Compt. Rend. Trav. Lab. Carlsberg. 38, 385–397 (1971)
Y. Pouliot, M.-M. Guy, M. Tremblay, A.-C. Gaonac’h, B.P.C.P. Ting, S.F. Gauthier, N. Voyer, J. Agric. Food Chem. 57, 3760–3764 (2009)
N. Creusot, H. Gruppen, J. Agric. Food Chem. 56, 10332–10339 (2008)
M.E. Bruins, N. Creusot, H. Gruppen, A.E.M. Janssen, R.M. Boom, J. Agric. Food Chem. 57, 5529–5534 (2009)
M.M. Vorob’ev, V. Vogel, W. Mäntele, Int. Dairy J. 30, 33–38 (2013)
F.C. Church, H.C. Swaisgood, D.H. Porter, G.L. Catignani, J. Dairy Sci. 66, 1218–1227 (1983)
J. Maurer, S. Haselbach, O. Klein, D. Baykut, V. Vogel, W. Mäntele, J. Am. Chem. Soc. 133, 1134–1140 (2011)
W. Heller, W.J. Pangonis, J. Chem. Phys. 26, 498–506 (1957)
S.H. Maron, P.E. Pierce, M.E. Elder, J. Macromol. Sci. B. 1, 29–39 (1967)
M. Dalgalarrondo, E. Dufor, J.-M. Chobert, C. Bertrand-Harb, T. Haertle, Int. Dairy J. 5, 1–14 (1995)
T. Sato, A. Chiba, R. Nozaki, J. Chem. Phys. 110, 2508–2521 (1999)
G. Güler, E. Džafić, M.M. Vorob’ev, V. Vogel, W. Mäntele, Spectrochim. Acta A 79, 104–111 (2011)
N. Creusot, H. Gruppen, J. Agric. Food Chem. 55, 9241–9250 (2007)
D. Rivera-Burgos, F.E. Regnier, Anal. Chem. 84, 7021–7028 (2012)
Acknowledgments
This research was supported in part by a research fellowship from the Deutscher Akademischer Austauschdienst (DAAD) to M.M.V. The authors would like to thank Jürgen Mauer (Institut für Bio-physik) for his valuable contributions to the development of the light scattering setup.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vorob’ev, M.M., Strauss, K., Vogel, V. et al. Demasking of Peptide Bonds During Tryptic Hydrolysis of β-casein in the Presence of Ethanol. Food Biophysics 10, 309–315 (2015). https://doi.org/10.1007/s11483-015-9391-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-015-9391-6