Abstract
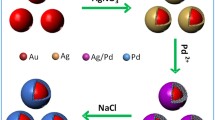
In this work, we demonstrated a simple and efficacious two-step method for the synthesis of Ag@Au core–shell nanoparticles (Ag@AuNPs) and the Ag/Au hollow nanocages (Ag/AuNCs) with Ag nanoparticles (AgNPs) as seeds by adjusting pH, and the preparation of hybrid Ag@AuNPs- or Ag/AuNCs-graphene oxide nanocomposites (Ag@AuNPs-GO or Ag/AuNCs-GO) based on the self-assembly. It was noticed from the electrostatic assembly experiment that the loading amount of Ag/AuNCs on GO nanosheet was more than that of Ag@AuNPs. The as-synthesized hybrid materials were characterized by transmission electron microscopy, atomic force microscopy, ζ-potential, high-angle annular dark-field scanning transmission electron microscopy, thermogravimetric analyzer and X-ray diffraction. Catalytic activities of Ag@AuNPs, Ag/AuNCs and Ag/AuNCs-GO nanostructures were investigated in the reduction of 4-, 3- or 2-nitrophenol to 4-, 3- or 2-aminophenol, and on the basis of comparative kinetic studies the following trend was obtained for the related catalytic activity: Ag/AuNCs-GO > Ag/AuNCs > Ag@AuNPs. These observations were attributed to the simultaneous effects of surface area available for catalytic reaction and composition of the hybrid nanostructures.
摘要
本文介绍了一种简单有效的制备银核金壳核壳纳米粒子(Ag@AuNPs)和银/金中空纳米笼(Ag/AuNCs)的合成方法,该方法分为两步:首先合成银纳米粒子(AgNPs),然后以该银纳米粒子为种子,通过调整溶液的pH,实现上述两种纳米材料的可控合成。之后基于自组装的方法制备得到了银金核壳纳米粒子-氧化石墨烯纳米复合物(Ag@AuNPs-GO)及银/金中空纳米笼-氧化石墨烯纳米复合物(Ag/AuNCs-GO)。研究发现,利用静电自组装的方法,Ag/AuNCs比Ag@AuNPs更容易组装到氧化石墨烯上。利用TEM, AFM, ζ-电位,HAADF-STEM,TGA及XRD等方法对所制备的杂化材料进行了表征。另外,研究了Ag@AuNPs, Ag/AuNCs 和Ag/AuNCs-GO 3种纳米材料在催化4-, 3-或2-硝基苯酚还原为4-, 3-或2-硝基苯胺方面所表现出的催化性质,对比研究发现其催化活性具有如下趋势:Ag/AuNCs-GO > Ag/AuNCs > Ag@AuNPs。实验结果表明,纳米杂化材料的组分和其提供的有效表面积都对其优异的催化活性起了重要作用。








Similar content being viewed by others
References
Sun YG, Xia YN (2002) Shape-controlled synthesis of gold and silver nanoparticles. Science 298:2176–2179
Li X, Song J, Chen BL et al (2016) A label-free colorimetric assay for detection of c-Myc mRNA based on peptide nucleic acid and silver nanoparticles. Sci Bull 61:276–281
Zhang LF, Zhong SL, Xu AW (2013) Highly branched concave Au/Pd bimetallic nanocrystals with superior electrocatalytic activity and highly efficient SERS enhancement. Angew Chem Int Ed 52:645–649
Zhang S, Shao YY, Liao HG et al (2011) Graphene decorated with PtAu alloy nanoparticles: facile synthesis and promising application for formic acid oxidation. Chem Mater 23:1079–1081
Ghosh SK, Mandal M, Kundu S et al (2004) Microwave-assisted facile green synthesis of silver nanoparticles and spectroscopic investigation of the catalytic activity. Appl Catal A 268:61–66
Zhang JW, Hou CP, Huang H et al (2013) Surfactant-concentration-dependent shape evolution of Au–Pd alloy nanocrystals from rhombic dodecahedron to trisoctahedron and hexoctahedron. Small 9:538–544
Tao F, Grass ME, Zhang YW et al (2008) Reaction-driven restructuring of Rh–Pd and Pt–Pd core–shell nanoparticles. Science 322:932–934
Lu Y, Yuan JY, Polzer F et al (2010) In situ growth of catalytic active Au–Pt bimetallic nanorods in thermoresponsive core–shell microgels. ACS Nano 4:7078–7086
Scott RWJ, Sivadinarayana C, Wilson OM (2005) Titania-supported PdAu bimetallic catalysts prepared from dendrimer-encapsulated nanoparticle precursors. J Am Chem Soc 127:1380–1381
Hong M, Xu LD, Wang FL et al (2016) In Situ synthesized Au–Ag nanocages on graphene oxide nanosheets: a highly active and recyclable catalyst for the reduction of 4-nitrophenol. New J Chem 40:1685–1692
Grzelczak M, Perez-Juste J, de Abajo FJG (2007) Optical properties of platinum-coated gold nanorods. J Phys Chem C 111:6183–6188
Tsao YC, Rej S, Chiu CY (2014) Aqueous phase synthesis of Au–Ag core–shell nanocrystals with tunable shapes and their optical and catalytic properties. J Am Chem Soc 136:396–404
Chen CH, Subramanyam L, Chen JM et al (2007) Fe L-edge X-ray absorption spectroscopy of low-spin heme relative to non-heme Fe complexes: delocalization of Fe d-electrons into the porphyrin ligand. J Am Chem Soc 1:113–125
Zhang QB, Xie JP, Lee JY et al (2008) Synthesis of Ag@AgAu metal core/alloy shell bimetallic nanoparticles with tunable shell compositions by a galvanic replacement reaction. Small 4:1067–1071
Zeng J, Zhang Q, Chen J et al (2010) A comparison study of the catalytic properties of Au-based nanocages, nanoboxes and nanoparticles. Nano Lett 10:30–35
Skrabalak SE, Chen JY, Sun YG et al (2008) Gold nanocages: synthesis, properties, and applications. Acc Chem Res 41:1587–1595
Novoselov KS, Geim AK, Morozov SV et al (2004) Electric field Effect in atomically thin carbon films. Science 306:666–669
Bai S, Shen XP (2012) Graphene–inorganic nanocomposites. RSC Adv 2:64–98
Seselj N, Engelbrekt C, Zhang JD et al (2015) Graphene-supported platinum catalysts for fuel cells. Sci Bull 60:864–876
Li YJ, Gao W, Ci LJ (2010) Catalytic performance of Pt nanoparticles on reduced graphene oxide for methanol electro-oxidation. Carbon 48:1124–1130
Guo SJ, Dong SJ, Wang EK (2010) Three-dimensional Pt-on-Pd bimetallic nanodendrites supported on graphene nanosheet: facile synthesis and used as an advanced nanoelectrocatalyst for methanol oxidation. ACS Nano 4:547–555
Choi Y, Bae HS, Seo S (2011) Hybrid gold nanoparticle-reduced graphene oxide nanosheets as active catalysts for highly efficient reduction of nitroarenes. J Mater Chem 21:15431–15436
Li HS, Xia H, Ding WC et al (2014) Synthesis of monodisperse, quasi-spherical silver nanoparticles with sizes defined by the nature of silver precursors. Langmuir 30:2498–2504
Yang Y, Liu JY, Fu ZW et al (2014) Galvanic replacement-free deposition of Au on Ag for core–shell nanocubes with enhanced chemical stability and SERS activity. J Am Chem Soc 136:8153–8156
Jia YY, Cao ZM, Chen QL et al (2015) Synthesis of composition-tunable octahedral Pt–Cu alloy nanocrystals by controlling reduction kinetics of metal precursors. Sci Bull 60:1002–1008
Personick M, Mirking CA (2013) Making sense of the mayhem behind shape control in the synthesis of gold nanoparticles. J Am Chem Soc 135:18238–18247
Choi Y, Hong S, Liu L et al (2012) Galvanically replaced hollow Au-Ag nanospheres: study of their surface plasmon resonance. Langmuir 28:6670–6676
Dong HF, Gao WC, Yan F et al (2010) Fluorescence resonance energy transfer between quantum dots and graphene oxide for sensing biomolecules. Anal Chem 82:5511–5517
Gittins DI, Caruso F (2001) Spontaneous phase transfer of nanoparticulate metals from organic to aqueous media. Angew Chem Int Ed 40:3001–3004
Liang M, Su RX, Huang RL (2014) Facile in situ synthesis of silver nanoparticles on procyanidin-grafted eggshell membrane and their catalytic properties. ACS Appl Mater Interfaces 6:4638–4649
Du Y, Chen HL, Chen RZ et al (2004) Synthesis of p-aminophenol from p-nitrophenol over nano-sized nickel catalysts. Appl Catal A 277:259–264
Zhang ZY, Shao CL, Zou P et al (2011) In situ assembly of well-dispersed gold nanoparticles on electrospun silica nanotubes for catalytic reduction of 4-nitrophenol. Chem Commun 47:3906–3908
Deng YH, Cai Y, Sun ZK et al (2010) Multifunctional mesoporous composite microspheres with well-designed nanostructure: a highly integrated catalyst system. J Am Chem Soc 132:8466–8473
Jiang HL, Akita T, Ishida T et al (2011) Synergistic catalysis of Au@Ag core–shell nanoparticles stabilized on metal–organic framework. J Am Chem Soc 133:1304–1306
Hayakawa K, Yoshimura T, Esumi K (2003) Preparation of gold–dendrimer nanocomposites by laser irradiation and their catalytic reduction of 4-nitrophenol. Langmuir 19:5517–5521
Gangula A, Podila R, Ramakrishna M et al (2011) Catalytic reduction of 4-nitrophenol using biogenic gold and silver nanoparticles derived from breynia rhamnoides. Langmuir 27:15268–15274
Bai S, Shen XP, Zhu GX et al (2012) In situ growth of Ni x Co100−x ganoparticles on reduced graphene oxide nanosheets and their magnetic and catalytic properties. ACS Appl Mater Interfaces 4:2378–2386
Barman BK, Nanda KK (2013) The dual role of Zn-acid medium for one-step rapid synthesis of M@rGO (M = Au, Pt, Pd and Ag) hybrid nanostructures at room temperature. Chem Commun 49:8949–8951
Liang M, Wang L, Su R et al (2013) Synthesis of silver nanoparticles within cross-linked lysozyme crystals as recyclable catalysts for 4-nitrophenol reduction. Catal Sci Technol 3:1910–1914
Chen XM, Cai ZX, Chen X et al (2014) AuPd bimetallic nanoparticles decorated on graphene nanosheets: their green synthesis, growth mechanism and high catalytic ability in 4-nitrophenol reduction. J Mater Chem A 2:5668–5674
Wu HX, Wang P, He HL et al (2012) Controlled synthesis of porous Ag/Au bimetallic hollow nanoshells with tunable plasmonic and catalytic properties. Nano Res 5:135–144
Mahmoud MA, Narayanan R, El-sayed MA (2013) Enhancing colloidal metallic nanocatalysis: sharp edges and corners for solid nanoparticles and cage effect for hollow ones. Acc Chem Res 46:1795–1805
Zhang YW, Liu S, Lu WB et al (2011) In situ green synthesis of Au nanostructures on graphene oxide and their application for catalytic reduction of 4-nitrophenol. Catal Sci Technol 1:1142–1144
Ji ZY, Shen XP, Zhu GX et al (2012) Reduced graphene oxide/nickel nanocomposites: facile synthesis, magnetic and catalytic properties. J Mater Chem 22:3471–3477
Rothenberg G (2008) Catalysis: concepts and green application. Wiley-VCH, Weinheim, p 11
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21105042), the Science Foundation of China Postdoctor (2014M560572), the Natural Science Foundation of Shandong Province (ZR2015BM024), and Tai-Shan Scholar Research Fund of Shandong Province. The study was partially supported by grant NIH 1R01DA037838 to Drs. Li and Nair.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Xu, L., Hong, M., Wang, Y. et al. Tunable synthesis solid or hollow Au–Ag nanostructure, assembled with GO and comparative study of their catalytic properties. Sci. Bull. 61, 1525–1535 (2016). https://doi.org/10.1007/s11434-016-1165-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-016-1165-0