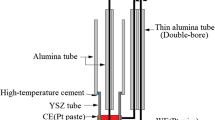
Abstract
This work presents a comprehensive study for the electrochemical behaviors of zirconium in LiCl-KCl eutectic. The effects of stirring, temperature and Zr concentration on the electrode reactions, the ZrCl4 sublimation from the melt, microcosmic morphologies of Zr deposits (ZrCl and Zr) obtained at different potential and temperature have been investigated. The behaviors of Zr(IV), on a large concentration range from 0.13% to 2.28% in melt, show a multiple-step reaction involving Zr(IV), Zr(II), ZrCl and Zr species. Temperature plays a crucial role on the changes of Zr(IV) reduction behavior on the solid electrode. The Zr(IV)/ZrCl couple is more easily observed at lower temperature and gradually diminishes with the increase of temperature. The Zr(IV)/Zr(II) and Zr(II)/Zr reactions are predominant on the W electrode at higher temperatures. At 673 K, a layered structure of insoluble ZrCl formed by potentiostatic electrolyses at 1.1 V was visualized by scanning electron microscopy-energy dispersive X-ray (SEM-EDS), while only Zr metal particles was observed at higher temperature than 773 K. An evolution of the Zr-based structure and size corresponding to the ZrCl and Zr metal based on different potentiostatic electrolysis was observed. The average particle size of the Zr metalparticles increases with the increase of temperature.
Similar content being viewed by others
References
Kinoshita K, Koyama T, Inoue T, Ougier M, Glatz JP. J Phys Chem Solids, 2005, 66: 619–624
Luo J, Wang C, Lan J, Wu Q, Zhao Y, Chai Z, Nie C, Shi W. Sci China Chem, 2016, 59: 324–331
Zhang Y, Lan J, Wu Q, Wang C, Bo T, Chai Z, Shi W. Sci China Chem, 2015, 58: 1891–1897
Murakami T, Sakamura Y, Akiyama N, Kitawaki S, Nakayoshi A, Fukushima M. J Nucl Mater, 2011, 414: 194–199
Ahluwalia RK, Hua TQ, Geyer HK. Nucl Technol, 2001, 133: 103–118
Koyama T, Iizuka M. Pyrochemical reprocessing of used nuclear fuels. In: 218th ECS Meeting. Las Vegas, 2010
Simpson MF, Law JD. Nuclear Fuel Reprocessing. U.S. Department of Energy Office of Nuclear Energy. Idaho Falls, 2010
Soucek P, Murakami T, Claux B, Meier R, Malmbeck R, Tsukada T. J Nucl Mater, 2015, 459: 114–121
Ghosh S, Vandarkuzhali S, Gogoi N, Venkatesh P, Seenivasan G, Reddy BP, Nagarajan K. Electrochim Acta, 2011, 56: 8204–8218
Iizuka M, Omori T, Tsukada T. J Nucl Sci Tech, 2010, 47: 244–254
Soucek P, Cassayre L, Malmbeck R, Mendes E, Jardin R, Glatz J P. Radiochim Acta, 2008, 96: 315–322
Iizuka M, Kinoshita K, Koyama T. J Phys Chem Solids, 2005, 66: 427–432
Fabian CP, Luca V, Le TH, Bond AM, Chamelot P, Massot L, Caravaca C, Hanley TL, Lumpkin GR. J Electrochem Soc, 2013, 160: H81–H86
Sakamura Y. J Electrochem Soc, 2004, 151: C187
Lee CH, Kang KH, Jeon MK, Heo CM, Lee YL. J Electrochem Soc, 2012, 159: D463–D468
Park J, Choi S, Sohn S, Kim KR, Hwang IS. J Electrochem Soc, 2014, 161: H97–H104
Lee CH, Lee YL, Jeon MK, Choi YT, Kang KH, Park GI, Park KT. ECS Trans, 2014, 64: 609–615
Baboian R, Hill DL, Bailey RA. J Electrochem Soc, 1965, 112: 1221
Ghosh S, Vandarkuzhali S, Venkatesh P, Seenivasan G, Subramanian T, Prabhakara Reddy B, Nagarajan K. J Electroanal Chem, 2009, 627: 15–27
Hoover RO, Yoon D, Phongikaroon S. J Nucl Mater, 2016, 476: 179–187
Cai Y, Liu H, Xu Q, Song Q, Liu H. RSC Adv, 2015, 5: 31648–31655
Gibilaro M, Massot L, Chamelot P, Cassayre L, Taxil P. Electrochim Acta, 2013, 95: 185–191
Groult H, Barhoun A, El Ghallali H, Borensztjan S, Lantelme F. J Electrochem Soc, 2008, 155: e19
Basile F, Chassaing E, Lorthioir G. J Appl Electrochem, 1981, 11: 645–651
Xu L, Xiao Y, Xu Q, van Sandwijk A, Li J, Zhao Z, Song Q, Yang Y. RSC Adv, 2016, 6: 84472–84479
Goto T, Ishigaki H, Ito Y. Mater Sci Eng-A, 2004, 371: 353–358
Lee CH, Jeon MK, Heo CM, Lee YL, Kang KH, Park GI. J Electrochem Soc, 2012, 159: e171–E176
Tylka MM, Willit JL, Prakash J, Williamson MA. J Electrochem Soc, 2015, 162: H625–H633
Acknowledgments
This work was supported by the National Natural Science Foundation of China (91426302, 91126006, 91326202) and the “Strategic Priority Research program” of the Chinese Academy of Sciences (XDA030104).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Liu, K., Liu, YL., Pang, JW. et al. Condition dependence of Zr electrochemical reactions and morphological evolution of Zr deposits in molten salt. Sci. China Chem. 60, 264–274 (2017). https://doi.org/10.1007/s11426-016-0321-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-016-0321-x