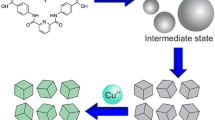
Abstract
The morphology and structure of zinc oxide (ZnO), one of the important semiconductors, are relevant to its properties and applications. The preparation of ZnO with tunable morphology and desired structure is an attractive topic in the field of material synthesis. This work reports a facile method for the synthesis of ZnO with controllable morphology and crystal orientation using Zn-based coordination polymer particles (Zn-CPP) as precursors. Using hydrothermal method, Zn-CPP with morphologies of microrod, nanoplate, flower-like, arrow-tipped microsheet, and square cylinder were successfully synthesized via the coordination between metal ions Zn2+ and organic ligand 1,4,5,8-naphthalenetetracarboxylic dianhydride in aqueous solution. Subsequent thermal treatment of the Zn-CPP successfully resulted in the formation of porous ZnO with similar morphology to Zn-CPP. It is also found that the ZnO with enhanced (002) orientation could be obtained from Zn-CPP with preferred (002) orientation. This strategy could be extended for the preparation of other metal oxides with desired shape and structure.
Similar content being viewed by others
References
Krishnan B, Irimpan L, Nampoor VPN, Kumar V. Synthesis and nonlinear optical studies of nano ZnO colloids. Physica E, 2008, 40: 2787–2790
Tokumoto MS, Pulcinelli SH, Santilli CV, Briois V. Catalysis and temperature dependence on the formation of ZnO nanoparticles and of zinc acetate derivatives prepared by the Sol-Gel route. J Phys Chem B, 2003, 107: 568–574
Zi M, Zhu M, Chen L, Wei H, Yang X, Cao B. ZnO photoanodes with different morphologies grown by electrochemical deposition and their dye-sensitized solar cell oroperties. Ceram Int, 2014, 40: 7965–7970
Huang MH, Mao S, Feick H, Yan HQ, Wu YY, Kind H, Weber E, Russo R, Yang PD. Room-temperature ultraviolet nanowire nanolasers. Science, 2001, 292: 1897–1899
Goldberger J, Sirbuly DJ, Law M, Yang P. ZnO nanowire transistors. J Phys Chem B, 2005, 109: 9–14
Kind H, Yan HQ, Messer B, Law M, Yang PD. Nanowire ultraviolet photodetectors and optical switches. Adv Mater, 2002, 14: 158–160
Wan Q, Li QH, Chen YJ, Wang TH, He XL, Li JP, Lin CL. Fabrication and ethanol sensing characteristics of Zno nanowire gas sensors. Appl Phys Lett, 2004, 84: 3654–3656
Wang ZL, Song JH. Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science, 2006, 312: 242–246
Sakthivel S, Neppolian B, Shankar MV, Arabindoo B, Palanichamy M, Murugesan V. Solar photocatalytic degradation of azo dye: comparison of photocatalytic efficiency of ZnO and TiO2. Sol Energy Mater Sol Cells, 2003, 77: 65–82
McLaren A, Valdes-Solis T, Li G, Tsang SC. Shape and size effects of ZnO nanocrystals on photocatalytic activity. J Am Chem Soc, 2009, 131: 12540–12541
Xie X, Li Y, Liu ZQ, Haruta M, Shen W. Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature, 2009, 458: 746–749
Zhou X, Lan J, Liu G, Deng K, Yang Y, Nie G, Yu J, Zhi L. Facet-mediated photodegradation of organic dye over hematite architectures by visible light. Angew Chem Int Ed, 2012, 51: 178–182
Lou XW, Wang Y, Yuan C, Lee JY, Archer LA. Template-free synthesis of SnO2 hollow nanostructures with high lithium storage capacity. Adv Mater, 2006, 18: 2325–2329
Andelman T, Gong YY, Polking M, Yin M, Kuskovsky I, Neumark G, O’Brien S. Morphological control and photoluminescence of zinc oxide nanocrystals. J Phys Chem B, 2005, 109: 14314–14318
Lyu SC, Zhang Y, Lee CJ, Ruh H, Lee HJ. Low-temperature growth of ZnO nanowire array by a simple physical vapor-deposition method. Chem Mater, 2003, 15: 3294–3299
Li Y, Meng GW, Zhang LD, Phillipp F. Ordered semiconductor ZnO nanowire arrays and their photoluminescence properties. Appl Phys Lett, 2000, 76: 2011–2013
Yin M, Gu Y, Kuskovsky IL, Andelman T, Zhu Y, Neumark GF, O’Brien S. Zinc oxide quantum rods. J Am Chem Soc, 2004, 126: 6206–6207
Li JY, Chen XL, Li H, He M, Qiao ZY. Fabrication of zinc oxide nanorods. J Cryst Growth, 2001, 233: 5–7
Guo L, Yang SH, Yang CL, Yu P, Wang JN, Ge WK, Wong GKL. Synthesis and characterization of poly(Vinylpyrrolidone)-modified zinc oxide nanoparticles. Chem Mater, 2000, 12: 2268–2274
Meulenkamp EA. Synthesis and growth of ZnO nanoparticles. J Phys Chem B, 1998, 102: 5566–5572
Cozzoli PD, Curri ML, Agostiano A, Leo G, Lomascolo M. ZnO Nanocrystals by a non-hydrolytic route: synthesis and characterization. J Phys Chem B, 2003, 107: 4756–4762
Pan ZW, Dai ZR, Wang ZL. Nanobelts of semiconducting oxides. Science, 2001, 291: 1947–1949
Chen Z, Shan ZW, Cao MS, Lu L, Mao SX. Zinc oxide nanotetrapods. Nanotechnology, 2004, 15: 365–369
Yan HQ, He RR, Pham J, Yang PD. Morphogenesis of one-dimensional ZnO nano- and microcrystals. Adv Mater, 2003, 15: 402–405
Jung S, Cho W, Lee HJ, Oh M. Self-template-directed formation of coordination-polymer hexagonal tubes and rings, and their calcination to ZnO rings. Angew Chem Int Ed, 2009, 48: 1459–1462
Cho W, Park S, Oh M. Coordination polymer nanorods of Fe-MIL-88B and their utilization for selective preparation of hematite and magnetite nanorods. Chem Commun, 2011, 47: 4138–4140
Zhao J, Li M, Sun J, Liu L, Su P, Yang Q, Li C. Metal-oxide nanoparticles with desired morphology inherited from coordination-polymer precursors. Chem-Eur J, 2012, 18: 3163–3168
Cho W, Lee YH, Lee HJ, Oh M. Systematic transformation of coordination polymer particles to hollow and non-hollow In2O3 with pre-defined morphology. Chem Commun, 2009, 4756-4758
Cho W, Lee YH, Lee HJ, Oh M. Multi ball-in-ball hybrid metal oxides. Adv Mater, 2011, 23: 1720–1723
Koner R, Goldberg I. Supramolecular reactivity of naphthalene-1,4,5, 8-tetracarboxylic acid towards transition metal ions: coordination polymers and discrete complexes with Cu-II, Ni-II and Co-II. Cryst Eng Comm, 2009, 11: 367–374
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Su, P., Zhao, J., Rong, F. et al. Fabrication of ZnO with tunable morphology through a facile treatment of Zn-based coordination polymers. Sci. China Chem. 58, 411–416 (2015). https://doi.org/10.1007/s11426-014-5290-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-014-5290-9