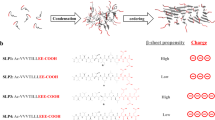
Abstract
Numerous peptides derived from naturally occurring proteins or de novo designed have been found to self-assemble into various nanostructures. These well-defined nanostructures have shown great potential for a variety of biomedical and biotechnological applications. In particular, surfactant-like peptides (SLPs) have distinctive advantages in their length, aggregating ability, and water solubility. In this article, we report recent advances in the mechanistic understanding of the self-assembly principles of SLPs and in their applications, most of which have been made in our laboratory. Hydrogen bonding between peptide backbones, hydrophobic interaction between hydrophobic side chains, and electrostatic repulsion between charged head groups all have roles in mediating the self-assembly of SLPs; the final self-assembled nanostructures are therefore dependent on their interplay. SLPs have shown diverse applications ranging from membrane protein stabilization and antimicrobial/anticancer agents to nanofabrication and biomineralization. Future advances in the self-assembly of SLPs will hinge on their large-scale production, the design of new functional SLPs with targeted properties, and the exploitation of new or improved applications.
Similar content being viewed by others
References
Whitesides GM, Grzybowski B. Self-assembly at all scales. Science, 2002, 295: 2418–2421
Whitesides GM, Boncheva M. Beyond molecules: self-assembly of mesoscopic and macroscopic components. Proc Natl Acad Sci USA, 2002, 99: 4769–4774
Dobson CM. Protein folding and misfolding. Nature, 2003, 426: 884–890
Winfree E, Liu F, Wenzler LA, Seeman NC. Design and self-assembly of two-dimensional DNA crystals. Nature, 1998, 394: 539–544
Rothemund PWK. Folding DNA to create nanoscale shapes and patterns. Nature, 2006, 440: 297–302
Ghadiri MR, Granja JR, Milligan RA, McRee DE, Khazanovich N. Self-assembling organic nanotubes based on a cyclic peptide archi tecture. Nature, 1993, 366: 324–347
Zhang S, Holmes T, Lockshin C, Rich A. Spontaneous assembly of a self-complementary oligopeptide to form a stable macroscopic membrane. Proc Natl Acad Sci USA, 1993, 90: 3334–3338
Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science, 2001, 294: 1684–1688
Reches M, Gazit E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science, 2003, 300: 625–627
Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotech, 2003, 21: 1171–1178
Schnur JM. Lipid tubules: a paradigm for molecularly engineered structures. Science, 1993, 262: 1669–1676
Gazit E. Self-assembled peptide nanostructures: the design of molecular building blocks and their technological utilization. Chem Soc Rev, 2007, 36: 1263–1269
Zhao X, Zhang S. Molecular designer self-assembling peptides. Chem Soc Rev, 2006, 35: 1105–1110
Ulijn RV, Smith AM. Designing peptide based nanomaterials. Chem Soc Rev, 2008, 37: 664–675
Zhao X, Pan F, Xu H, Yaseen M, Shan H, Hauser CAE, Zhang S, Lu JR. Molecular self-assembly and applications of designer peptide amphiphiles. Chem Soc Rev, 2010, 39: 3480–3498
Cui H, Webber MJ, Stupp SI. Self-assembly of peptide amphiphiles: from molecules to nanostructures to biomaterials. Biopolymers, 2010, 94: 1–18
Yan X, Zhu P, Li J. Self-assembly and application of diphenylalanine-based nanostructures. Chem Soc Rev, 2010, 39: 1877–1890
Hamley IW. Self-assembly of amphiphilic peptides. Soft Matter, 2011, 7: 4122–4138
Loo Y, Zhang S, Hauser CAE. From short peptides to nanofibers to macromolecular assemblies in biomedicine. Biotechnol Adv, 2012, 30: 593–603
Hosseinkhani H, Hong PD, Yu DS. Self-assembled proteins and peptides for regenerative medicine. Chem Rev, 2013, 113: 4837–4861
Fichman G, Gazit E. Self-assembly of short peptides to form hydrogels: design of building blocks, physical properties and technological applications. Acta Biomater, 2014, 10: 1671–1682
Ghadiri MR, Granja JR, Buehler LK. Artificial transmembrane ion channels from self-assembling peptide nanotubes. Nature, 1994, 369: 301–304
Fernandez-Lopez S, Kim HS, Choi EC, Delgado M, Granja JR, Khasanov A, Kraehenbuehl K, Long G, Weinberger DA, Wilcoxen KM, Ghadiri MR. Antibacterial agents based on the cyclic D,L-α-peptide architecture. Nature, 2001, 412: 452–455
Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, Grodzinsky AJ. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci USA, 2002, 99: 9996–10001
Holmes TC, de Lacalle S, Su X, Liu G, Rich A, Zhang S. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc Natl Acad Sci USA, 2000, 97: 6728–6733
Koutsopoulos S, Unsworth LD, Nagai Y, Zhang S. Controlled release of functional proteins through designer self-assembling peptide nanofiber hydrogel scaffold. Proc Natl Acad Sci USA, 2009, 106: 4623–4628
Aggeli A, Bell M, Boden N, Keen JN, Knowles PF, McLeish TCB, Pitkeathly M, Radford SE. Responsive gels formed by the spontaneous self-assembly of peptides into polymeric β-sheet tapes. Nature, 1997, 386: 259–262
Collier JH, Messersmith PB. Enzymatic modification of self-assembled structures with tissue transglutaminase. Bioconjugate Chem, 2003, 14: 748–755
Hartgerink JD, Beniash E, Stupp SI. Peptide-amphiphile nanofibers: a versatile scaffold for the preparation of self-assembling materials. Proc Natl Acad Sci USA, 2002, 99: 5133–5138
Cui H, Muraoka T, Cheetham AG, Stupp SI. Self-assembly of giant peptide nanobelts. Nano Lett, 2009, 9: 945–952
Zhang S, Greenfield MA, Mata A, Palmer LC, Bitton R, Mantei JR, Aparicio C, de la Cruz MO, Stupp SI. A self-assembly pathway to aligned monodomain gels. Nat Mater, 2010, 9: 594–601
Makovitzki A, Baram J, Shai Y. Antimicrobial lipopolypeptides composed of palmitoyl di- and tricationic peptides: in vitro and in vivo activities, self-assembly to nanostructures, and a plausible mode of action. Biochemistry, 2008, 47: 10630–10636
Schneider JP, Pochan DJ, Ozbas B, Rajagopal K, Pakstis L, Kretsinger J. Responsive hydrogels from the intramolecular folding and self-assembly of a designed peptide. J Am Chem Soc, 2002, 124: 15030–15037
Haines-Butterick L, Rajagopal K, Branco M, Salick D, Rughani R, Pilarz M, Lamm MS, Pochan DJ, Schneider JP. Controlling hydrogelation kinetics by peptide design for three-dimensional encapsulation and injectable delivery of cells. Proc Natl Acad Sci USA, 2007, 104: 7791–7796
Smith AM, Williams RJ, Tang C, Coppo P, Collins RF, Turner ML, Saiani A, Ulijn RV. Fmoc-diphenylalanine self assembles to a hydrogel via a novel architecture based on π-π interlocked β-sheets. Adv Mater, 2008, 20: 37–41
Lu K, Jacob J, Thiyagarajan P, Conticello VP, Lynn DG. Exploiting amyloid fibril lamination for nanotube self-assembly. J Am Chem Soc, 2003, 125: 6391–6393
Tao K, Wang J, Zhou P, Wang C, Xu H, Zhao X, Lu JR. Self-assembly of short Aβ (16-22) peptides: effect of terminal capping and the role of electrostatic interaction. Langmuir, 2011, 27: 2723–2730
Dong H, Paramonov SE, Aulisa L, Bakota EL, Hartgerink JD. Self-assembly of multidomain peptides: balancing molecular frustration controls conformation and nanostructures. J Am Chem Soc, 2007, 129: 12468–12472
Bakota E, Wang Y, Danesh FR, Hartgerink JD. Injectable multidomain peptide nanofiber hydrogel as a delivery agent for stem cell secretome. Biomacromolecules, 2011, 12: 1651–1657
Banwell EF, Abelardo ES, Adams DJ, Birchall MA, Corrigan A, Donald AM, Kirkland M, Serpell LC, Butler MF, Woolfson DN. Rational design and application of responsive α-helical peptide hydrogels. Nat Mater, 2009, 8: 596–600
Vauthey S, Santoso S, Gong H, Watson N, Zhang S. Molecular self-assembly of surfactant-like peptides to form nanotubes and nanovesicles. Proc Natl Acad Sci USA, 2002, 99: 5355–5360
Santoso S, Hwang W, Hartman H, Zhang S. Self-assembly of surfactant-like peptides with variable glycine tails to form nanotubes and nanovesicles. Nano Lett, 2002, 2: 687–691
von Maltzahn G, Vauthey S, Santoso S, Zhang S. Positively charged surfactant-like peptides self-assembly into nanostructures. Langmuir, 2003, 19: 4332–4337
Kiley P, Zhao X, Vaughn M, Baldo MA, Bruce BD, Zhang S. Self-assembling peptide detergents stabilize isolated photosystem I on a dry surface for an extended time. PLoS Biol, 2005, 3: e230
Koutsopoulos S, Kaiser L, Maria H, Eriksson M, Zhang S. Designer peptide surfactants stabilize diverse functional membrane proteins. Chem Soc Rev, 2012, 41: 1721–1728
Mishra A, Loo Y, Deng R, Chuah Y J, Hee HT, Ying JY, Hauser CAE. Ultrasmall natural peptides self-assemble to strong temperature-resistant helical fibers in scaffolds suitable for tissue engineering. Nano Today, 2011, 6: 232–239
Xu H, Wang Y, Ge X, Han S, Wang S, Zhou P, Shan H, Zhao X, Lu JR. Twisted nanotubes formed from ultrashort amphiphilic peptide I3K and their templating for the fabrication of silica nanotubes. Chem Mater, 2010, 22: 5165–5173
Bucak S, Cenker C, Nasir I, Olsson U, Zackrisson M. Peptide nanotube nematic phase. Langmuir, 2009, 25: 4262–4265
Nagai A, Nagai Y, Qu H, Zhang S. Dynamic behaviors of lipid-like self-assembling peptide A6D and A6K nanotubes. J Nanosci Nanotech, 2007, 7: 1–7
Yang SJ, Zhang S. Self-assembling behavior of designer lipid-like peptides. Supramol Chem, 2006, 18: 389–396
van Hell AJ, Costa CICA, Flesch FM, Sutter M, Jiskoot W, Crommelin DJA, Hennink WE, Mastrobattista E. Self-assembly of recom binant amphiphilic oligopeptides into vesicles. Biomacromolecules, 2007, 8: 2753–2761
Khoe U, Yang Y, Zhang S. Self-assembly of nanodonut structure from a cone-shaped designer lipid-like peptide surfactant. Langmuir, 2009, 25: 4111–4114
Wang J, Han S, Meng G, Xu H, Xia D, Zhao X, Schweins R, Lu JR. Dynamic self-assembly of surfactant-like peptides A6K and A9K. Soft Matter, 2009, 5: 3870–3878
Han S, Cao S, Wang Y, Wang J, Xia D, Xu H, Zhao X, Lu JR. Self-assembly of short peptide amphiphiles: the cooperative effect of hydrophobic interaction and hydrogen bonding. Chem Eur J, 2011, 17: 13095–13102
Han S, Xu W, Cao M, Wang J, Xia D, Xu H, Zhao X, Lu JR. Interfacial adsorption of cationic peptide amphiphiles: a combined study of in situ spectroscopic ellipsometry and liquid AFM. Soft Matter, 2012, 8: 645–653
Baumann MK, Textor M, Reimhult E. Understanding self-assembled amphiphilic peptide supramolecular structures form primary structure helix propensity. Langmuir, 2008, 24: 7645–7647
Hauser CAE, Deng R, Mishra A, Loo Y, Khoe U, Zhuang F, Cheong DW, Accardo A, Sullivan MB, Riekel C, Ying JY, Hauser UA. Natural tri-to hexapeptides self-assemble in water to amyloid β-type fiber aggregates by unexpected α-helical intermediate structures. Proc Natl Acad Sci USA, 2011, 108: 1361–1366
Xu H, Wang J, Han S, Wang J, Yu D, Zhang H, Xia D, Zhao X, Waigh TA, Lu JR. Hydrophobic-region-induced transitions in self-assembled peptide nanostructures. Langmuir, 2009, 25: 4115–4123
Khoe U, Yang Y, Zhang S. Synergetic effect and hierarchical nanostructure formation in mixing two designer lipid-like peptide surfactants Ac-A6D-OH and Ac-A6K-NH2. Macromol Bio Sci, 2008, 8: 1060–1067
Zhao Y, Wang J, Deng L, Zhou P, Wang S, Wang Y, Xu H, Lu JR. Tuning the self-assembly of short peptides via sequence variations. Langmuir, 2013, 29: 13457–13464
Ge B, Yang F, Yu D, Liu S, Xu H. Designer amphiphilic short peptides enhance thermal stability of isolated photosystem-I. PLoS One, 2010, 5: e10233
Wang S, Ge X, Xue J, Fan H, Mu L, Li Y, Xu H, Lu JR. Mechanistic processes underlying biomimetic synthesis of silica nanotubes from self-assembled ultrashort peptide templates. Chem Mater, 2011, 23: 2466–2474
Wang S, Xue J, Ge X, Fan H, Xu H, Lu JR. Biomimetic synthesis of silica nanostructures with controllable morphologies and sizes through interfacial interactions. Chem Commun, 2012, 48: 9415–9417
Tao K, Wang J, Li Y, Xia D, Shan H, Xu H, Lu JR. Short peptide-directed synthesis of one-dimensional platinum nanostructures with controllable morphologies. Sci Rep, 2013, 3: 2565
Chen C, Pan F, Zhang S, Hu J, Cao M, Wang J, Xu H, Zhao X, Lu JR. Antibacterial activities of short designer peptides: a link between propensity for nanostructuring and capacity for membrane destabilization. Biomacromolecules, 2010, 11: 402–411
Chen C, Hu J, Zhang S, Zhou P, Zhao X, Xu H, Zhao X, Yaseen M, Lu JR. Molecular mechanism of antibacterial and antitumor actions of designer surfactant-like peptides. Biomaterials, 2012, 33: 592–603
Xu H, Chen C, Hu J, Zhou P, Zeng P, Cao C, Lu JR. Dual modes of antitumor action of an amphiphilic peptide A9K. Biomaterials, 2013, 34: 2731–2737
Hamley IW, Dehsorkhi A, Castellotto V. Self-assembled arginine-coated peptide nanosheets in water. Chem Commn, 2013, 49: 1850–1852
Dehsorkhi A, Castelletto V, Hamley IW. Interaction between a cationic surfactant-like peptide and lipid vesicles and its relationship to antimicrobial activity. Langmuir, 2013, 29: 14246–14253
Huang R, Qi W, Su R, Zhao J, He Z. Solvent and surface controlled self-assembly of diphenylalanine peptide: from microtubes to nanofibers. Soft Matter, 2011, 7: 6418–6421
Nagarajan R, Ruckenstein E. Theory of surfactant self-assembly: a predictive molecular thermodynamic approach. Langmuir, 1991, 7: 2934–2969
Daniel L, Minor J, Kim PS. Measurement of the β-sheet-forming propensities of amino acids. Nature, 1994, 367: 660–663
Nagarajan R. Molecular packing parameters and surfactant self-assembly: the neglected role of the surfactant tail. Langmuir, 2002, 18: 31–38
Cao M, Wang Y, Ge X, Cao C, Wang J, Xu H, Xia D, Zhao X, Lu JR. Effects of anions on nanostructuring of cationic amphiphilic peptides. J Phys Chem B, 2011, 115: 11862–11871
Zhao X, Nagai Y, Reeves PJ, Kiley P, Khorana HG, Zhang S. Designer short peptide surfactants stabilize G protein-coupled receptor bovine rhodopsin. Proc Natl Acad Sci USA, 2006, 103: 17707–17712
Wallin E, Heijne GV. Genome-wide analysis of integral membrane proteins from eubacterial, archaean, and eukaryotic organisms. Protein Sci, 1998, 7: 1029–1038
Loll PJ. Membrane protein structural biology: the high throughput challenge. J Struct Biol, 2003, 142:144–153
Nilsson J, Persson B, von Heijne G. Comparative analysis of amino acid distributions in integral membrane proteins from 107 genomes. Proteins, 2005, 60: 606–616
Zasloff M. Antimicrobial peptides of multicellular organisms. Nature, 2002, 415: 389–395
Dathe M, Wieprecht T. Structural features of helical antimicrobial peptides: their potential to modulate activity on model membranes and biological cells. BBA-Biomembranes, 1999, 1462: 71–87
Castelletto V, Hamley IW, Segarra-Maset MD, Gumbau CB, Miravet JF, Escuder B, Seitsonen J, Ruokolainen J. Tuning chelation by the surfactant-like peptide A6H using predetermined pH values. Biomacromolecules, 2014, 15: 591–598
Castelletto V, Gouveia RM, Connon CJ, Hamley IW, Seitsonen J, Nykänen A, Ruokolainen J. Alanine-rich amphiphilic peptide containing the RGD cell adhesion motif: a coating material for human fibroblast attachment and culture. Biomater Sci, 2014, 2: 362–369
Bakota EL, Aulisa L, Galler KM, Hartgerink JD. Enzymatic cross-linking of a nanofibrous peptide hydrogel. Biomacromolecules, 2011, 12: 82–87
Teixeira LSM, Feijen J, van Blitterswijk CA, Dijkstra PJ, Karperien M. Enzyme-catalyzed crosslinkable hydrogels: emerging strategies for tissue engineering. Biomaterials, 2012, 33: 1281–1290
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., Zhao, Y., Han, S. et al. Self-assembly of surfactant-like peptides and their applications. Sci. China Chem. 57, 1634–1645 (2014). https://doi.org/10.1007/s11426-014-5234-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-014-5234-4