Abstract
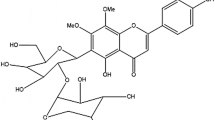
Acylated phenylethanoid glycosides, echinacoside (1) and acteoside (2), principal constituents in stems of Cistanche tubulosa (Orobanchaceae), inhibited the increase in postprandial blood glucose levels in starch-loaded mice at doses of 250–500 mg/kg p.o. These compounds (1 and 2) also significantly improved glucose tolerance in starch-loaded mice after 2 weeks of continuous administration at doses of 125 and/or 250 mg/kg/day p.o. without producing significant changes in body weight or food intake. In addition, several constituents from C. tubulosa, including 1 (IC50 = 3.1 μM), 2 (1.2 μM), isoacteoside (3, 4.6 μM), 2′-acetylacteoside (4, 0.071 μM), tubulosides A (5, 8.8 μM) and B (9, 4.0 μM), syringalide A 3-O-α-l-rhamnopyranoside (10, 1.1 μM), campneoside I (13, 0.53 μM), and kankanoside J1 (14, 9.3 μM), demonstrated potent rat lens aldose reductase inhibitory activity. In particular, the potency of compound 4 was similar to that of epalrestat (0.072 μM), a clinical aldose reductase inhibitor.

Similar content being viewed by others
References
Jiménez C, Riguera R (1994) Phenylethanoid glycosides in plants: structure and biological activity. Nat Prod Rep 11:591–606
Fu G, Pang H, Wong YH (2008) Naturally occurring phenylethanoid glycosides: potential leads for new therapeutics. Curr Med Chem 15:2592–2613
He J, Hu X-P, Zeng Y, Li Y, Wu H-Q, Qiu R-Z, Ma W-J, Li T, Li C-Y, He Z-D (2011) Advanced research on acteoside for chemistry and bioactivities. J Asian Nat Prod Res 13:449–464
Stoll A, Renz J, Brack A (1950) Isolierung und konstitution des echinacosids, eines glykosids aus den wurzeln von Echinacea angustifolia D. C. 6. mitteilung über antibakterielle stoffe. Helv Chim Acta 33:1877–1893
Becker H, Hsieh WC, Wylde R, Laffite C, Andary C (1982) Structure of echinacoside. Z Naturforsch C: Biosci 37C:351–353
Scarpati ML, Dell MF (1963) Isolation from Verbascum sinuatum of two new glucosides, verbascoside and isoverbascoside. Ann Chim 53:356–367
Birkofer L, Kaiser C, Thomas U (1968) Sugar esters. IV. acteoside and neoacteoside, sugar esters from Syringa vulgaris. Z Naturforsch, B: Chem Sci 23:1051–1058
Andary C, Wylde R, Laffite C, Privat G, Winternitz F (1982) Structures of varbascoside and orobanchoside, caffeic acid sugar esters from Orobanche rapum-genistae. Phytochemistry 21:1123–1127
Sakurai A, Kato T (1983) A new glycoside, kusaginin isolated from Clerodendron trichotomum. Bull Chem Soc Jpn 56:1573–1574
Lee KJ, Woo E-R, Choi CY, Shin DW, Lee DG, You HJ, Jeong HG (2004) Protective effect of acteoside on carbon tetrachloride-induced hepatotoxicity. Life Sci 74:1051–1064
Jia C, Shi H, Jin W, Zhang K, Jiang Y, Zhao M, Tu P (2009) Metabolism of echinacoside, a good antioxidant, in rats: isolation and identification of its biliary metabolites. Drug Metab Dispos 37:431–438
Jia Y, Guan Q, Guo Y, Du C (2012) Echinacoside stimulates cell proliferation and prevents cell apoptosis in intestinal epithelial MODE-K cells by up-regulation of transforming growth factor-β1 expression. J Pharmacol Sci 118:99–108
Li F, Yang Y, Zhu P, Chen W, Qi D, Shi X, Zhang C, Yang Z, Li P (2012) Echinacoside promotes bone regeneration by increasing OPG/RANKL ratio in MC3T3-E1 cells. Fitoterapia 83:1443–1450
Li F, Yang X, Yang Y, Guo C, Zhang C, Yang Z, Li P (2013) Antiosteoporotic activity of echinacoside in ovariectomized rats. Phytomedicine 20:549–557
Yoshikawa M, Matsuda H, Morikawa T, Xie H, Nakamura S, Muraoka O (2006) Phenylethanoid oligoglycosides and acylated oligosugars with vasorelaxant activity from Cistanche tubulosa. Bioorg Med Chem 14:7468–7475
Morikawa T, Pan Y, Ninomiya K, Imura K, Matsuda H, Yoshikawa M, Yuan D, Muraoka O (2010) Acylated phenylethanoid oligoglycosides with hepatoprotective activity from the desert plant Cistanche tubulosa. Bioorg Med Chem 18:1882–1890
Pan Y, Morikawa T, Ninomiya K, Imura K, Yuan D, Yoshikawa M, Muraoka O (2010) Bioactive constituents from Chinese natural medicines. XXXVI. Four new acylated phenylethanoid oligoglycosides, kankanosides J1, J2, K1, and K2, from stems of Cistanche tubulosa. Chem Pharm Bull 58:575–578
Xie H, Morikawa T, Matsuda H, Nakamura S, Muraoka O, Yoshikawa M (2006) Monoterpene constituents from Cistanche tubulosa: chemical structures of kankanosides A-E and kankanol. Chem Pharm Bull 54:669–675
Morikawa T, Pan Y, Ninomiya K, Imura K, Yuan D, Yoshikawa M, Hayakawa T, Muraoka O (2010) Iridoid and acyclic monoterpene glycosides, kankanosides L, M, N, O, and P from Cistanche tubulosa. Chem Pharm Bull 58:1403–1407
Kobayashi H, Oguchi H, Takizawa N, Miyase T, Ueno A, Usmanghani K, Ahmad M (1987) New phenylethanoid glycosides from Cistanche tubulosa (Schrenk) Hook. f. I. Chem Pharm Bull 35:3309–3314
Shimoda H, Tanaka J, Takahara Y, Takemoto K, Shan S-J, Su M-H (2009) The hypocholesterolemic effects of Cistanche tubulosa extract, a Chinese traditional crude medicine, in mice. Am J Chin Med 37:1125–1138
Yoshikawa M, Morikwa T, Matsuda H, Tanabe G, Muraoka O (2002) Absolute stereostructure of potent α-glucosidase inhibitor, salacinol, with unique thiosugar sulfonium sulfate inner salt structure from salacia reticulata. Bioorg Med Chem 10:1547–1554
Muraoka O, Morikawa T, Miyake S, Akaki J, Ninomiya K, Yoshikawa M (2010) Quantitative determination of potent α-glucosidase inhibitors, salacinol and kotalanol, in Salasia species using liquid chromatography-mass spectrometry. J Pharm Biomed Anal 52:770–773
Muraoka O, Morikawa T, Miyake S, Akaki J, Ninomiya K, Pongpiriyadacha Y, Yoshikawa M (2011) Quantitative analysis of neosalacinol and neokotalanol, another two potent α-glucosidase inhibitors from Salacia species, by LC-MS with ion pair chromatography. J Nat Med 65:142–148
Matsuda H, Morikawa T, Toguchida I, Yoshikawa M (2002) Structural requirements of flavonoids and related compounds for aldose reductase inhibitory activity. Chem Pharm Bull 50:788–795
Yoshikawa M, Morikawa T, Murakami T, Toguchida I, Harima S, Matsuda H (1999) Medicinal flowers. I. aldose reductase inhibitors and three new eudesmane-type sesquiterpenes, kikkanols A, B, and C, from the flowers of Chrysanthemum indicum L. Chem Pharm Bull 47:340–345
Matsuda H, Morikawa T, Toguchida I, Harima S, Yoshikawa M (2002) Medicinal flowers. VI. Absolute stereostructures of two new flavanone glycosides and a phenylbutanoid glycoside from the flowers of Chrysanthemum indicum L.: their inhibitory activities for rat lens aldose reductase. Chem Pharm Bull 50:972–975
Yoshikawa M, Murakami T, Ishiwada T, Morikawa T, Kagawa M, Higashi Y, Matsuda H (2002) New flavonol oligoglycosides and polyacylated sucroses with inhibitory effects on aldose reductase and platelet aggregation from the flowers of Prunus mume. J Nat Prod 65:1151–1155
Matsuda H, Morikawa T, Yoshikawa M (2002) Antidiabetogenic constituents from several natural medicines. Pure Appl Chem 74:1301–1308
Xie H, Wang T, Matsuda H, Morikawa T, Yoshikawa M, Tani T (2005) Bioactive constituents from Chinese natural medicines. XV. Inhibitory effect on aldose reductase and structures of saussureosides A and B from Saussurea medusa. Chem Pharm Bull 53:1416–1422
Morikawa T, Xie H, Wang T, Matsuda H, Yoshikawa M (2008) Bioactive constituents from Chinese natural medicines. XXXII. Aminopeptidase N and aldose reductase inhibitors from Sinocrassula indica: structures of sinocrassosides B4, B5, C1, and D1–D3. Chem Pharm Bull 56:1438–1444
Morikawa T, Chaipech S, Matsuda H, Hamao M, Umeda Y, Sato H, Tamura H, Kon’i H, Ninomiya K, Yoshikawa M, Pongpiriyadacha Y, Hayakawa T, Muraoka O (2012) Antidiabetogenic oligostilbenoids and 3-ethyl-4-phenyl-3,4-dihydroisocoumarins from the bark of Shorea roxburghii. Bioorg Med Chem 20:832–840
Morikawa T, Kishi A, Pongiriyadacha Y, Matusda H, Yoshikawa M (2003) Structures of new friedelane-type triterpenes and eudesmane-type sesquiterpene and aldose reductase inhibitors from Salacia chinensis. J Nat Prod 66:1191–1196
Acknowledgments
This work was supported in part by a Grant-in Aid for Scientific Research by Japan Society for the Promotion of Science (JSPS) KAKENHI a Grant Number 24590153 and The Japan–China Medical Association for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morikawa, T., Ninomiya, K., Imamura, M. et al. Acylated phenylethanoid glycosides, echinacoside and acteoside from Cistanche tubulosa, improve glucose tolerance in mice. J Nat Med 68, 561–566 (2014). https://doi.org/10.1007/s11418-014-0837-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-014-0837-9