Abstract
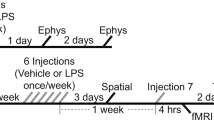
The aged brain is prone to excessive levels of immune activity, not initiated by an acute response to an extrinsic agent. While dietary melatonin is reported to attenuate the extent of expression of proinflammatory genes, little is known about the extent to which these changes can be translated into altered levels of corresponding proteins. The baseline levels of the proinflammatory cytokines, tumor necrosis factor alpha (TNF-α) and interleukin-1 alpha, were greater in older (~29 months old) compared to younger (~7 months old) mouse brains. Acute (3 h) exposure to lipopolysaccharide (LPS) induced activation of nuclear factor kappa B (NF-κB), but not inflammatory cytokines in the brain. The serum level of TNF-α was increased after LPS injection, indicating a systemic immune response to the bacterial cell wall component. Dietary melatonin (40 ppm for 9.3 weeks) did not prevent LPS-induced changes in younger animals but caused an increased systemic TNF-α response in older mice. Melatonin did reduce markers of carbonyl formation in brain proteins of young animals and nitrosylative damage to peptide-bound amino acid residues, in the brains of older animals. Acute LPS challenge did not significantly affect these oxidative markers. Thus, despite lack of clear evidence of attenuation of the NF-κB–cytokine inflammatory trajectory within the CNS by melatonin, this agent did show a protective effect against free radical-initiated injury to amino acid residues within proteins. The results illustrate that previously reported changes in gene expression following melatonin treatment need not be closely paralleled by corresponding changes in protein content.




Similar content being viewed by others
References
Akbulut KG, Gonul B, Akbulut H (2008) Exogenous melatonin decreases age-induced lipid peroxidation in the brain. Brain Res 1238:31–35
Baykal A, Iskit AB, Hamaloglu E, Guc MO, Hascelik G, Sayek I (2000) Melatonin modulates mesenteric blood flow and TNFα concentrations after lipopolysaccharide challenge. Eur J Surg 166:722–727
Bondy SC, Sharman EH (2010) Melatonin, oxidative stress and the aging brain. In: Bondy SC, Maiese K (eds) Oxidative stress in basic research and clinical practice: aging and age-related disorders. Humana, Totowa, pp 339–357
Brink TC, Regenbrecht C, Demetrius L, Lehrach H, Adjaye J (2009) Activation of the immune response is a key feature of aging mice. Biogerontology 10:721–734
Dkhar P, Sharma R (2011) Amelioration of age-dependent increase in protein carbonyls of cerebral hemispheres of mice by melatonin and ascorbic acid. Neurochem Int 59:996–1002
Escames G, López LC, Ortiz F, Ros E, Acuña-Castroviejo D (2006) Age-dependent lipopolysaccharide-induced iNOS expression and multiorgan failure in rats: effects of melatonin treatment. Exp Gerontol 41:1165–1173
Garcia-Macia M, Vega-Naredo I, de Gonzalo-Calvo D, Camello PJ, Camello-Almaraz C, Martin-Cano FE, Rodriguez-Colunga MJ, Pozo MJ, Coto-Montes AM (2010) Melatonin induces neural SOD2 expression independent of the NF-kappaB pathway and improves the mitochondrial population and function in old mice. J Pineal Res 50:54–63
Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR (2011) Melatonin-A pleiotropic orchestrating regulator molecule. Prog Neurobiol 93:350–384
Kornbrust DJ, Mavis RD (1980) Relative susceptibility of microsomes from lung, heart, liver, kidney, brain and testes to lipid peroxidation: correlation with vitamin E content. Lipids 15:315–322
Lahiri DK, Ge Y (2000) Electrophoretic mobility shift assay for the detection of specific DNA-protein complex in nuclear extracts from the cultured cells and frozen autopsy human brain tissue. Brain Res 5:257–265
Lahiri DK, Ge YW, Sharman EH, Bondy SC (2004) Age-related changes in serum melatonin in mice: higher levels of combined melatonin and 6-hydroxymelatonin sulfate in the cerebral cortex than serum, heart, liver and kidney tissues. J Pineal Res 36:217–223
Nava F, Calapai G, Facciolà G, Cuzzocrea S, Giuliani G, De Sarro A, Caputi AP (1997) Melatonin effects on inhibition of thirst and fever induced by lipopolysaccharide in rat. Eur J Pharmacol 331:267–274
Naidu KS, Morgan LW, Bailey MJ (2010) Inflammation in the avian spleen: timing is everything. BMC Mol Biol 11:104
Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT (2007) Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55:453–462
Raghavendra V, Agrewala JN, Kulkarni SK (2000) Melatonin reversal of lipopolysacharides-induced thermal and behavioral hyperalgesia in mice. Eur J Pharmacol 5:15–21
Reiter RJ, Acuna-Castroviejo D, Tan DX, Burkhardt S (2001) Free radical-mediated molecular damage: mechanisms for the protective actions of melatonin in the central nervous system. Ann NY Acad Sci 939:200–215
Sacco S, Aquilini L, Ghezzi P, Pinza M, Guglielmotti A (1998) Mechanism of the inhibitory effect of melatonin on tumor necrosis factor production in vivo and in vitro. Eur J Pharmacol 343:249–255
Santello FH, Frare EO, Caetano LC, AlonsoToldo MP, do Prado JC (2008) Melatonin enhances pro-inflammatory cytokine levels and protects against Chagas disease. J Pineal Res 45:79–85
Sharman KG, Sharman E, Bondy SC (2002) Dietary melatonin selectively reverses age-related changes in cortical basal cytokine mRNA levels, and their responses to an inflammatory stimulus. Neurobiol Aging 23:633–638
Sharman EH, Bondy SC, Sharman KZ, Lahiri D, Cotman CW, Perreau VM (2007) Effects of melatonin and age on gene expression in mouse CNS using microarray analysis. Neurochem Internat 50:336–344
Sharman EH, Sharman KZ, Bondy SC (2008) Melatonin causes gene expression in aged animals to respond to inflammatory stimuli in a manner differing from that of young animals. Curr Aging Sci 1:152–158
Shi D, Xiao X, Wang J, Liu L, Chen W, Fu L, Xie F, Huang W, Deng W (2012) Melatonin suppresses proinflammatory mediators in lipopolysaccharide-stimulated CRL1999 cells via targeting MAPK, NF-κB, c/EBPβ, and p300 signaling. J Pineal Res 53:154–165
Tresguerres JA, Kireev R, Forman K, Cuesta S, Tresguerres AF, Vara E (2012) Effect of chronic melatonin administration on several physiological parameters from old Wistar rats and SAMP8 mice. Curr Aging Sci 5:242–253
Tyagi E, Agrawal R, Nath C, Shukla R (2010) Effect of melatonin on neuroinflammation and acetylcholinesterase activity induced by LPS in rat brain. Eur J Pharmacol 640:206–210
Wu CC, Chiao CW, Hsiao G, Chen A, Yen MH (2001) Melatonin prevents endotoxin-induced circulatory failure in rats. J Pineal Res 30:147–156
Xu DX, Wang H, Ning H, Zhao L, Chen YH (2007) Maternally administered melatonin differentially regulates lipopolysaccharide-induced proinflammatory and anti-inflammatory cytokines in maternal serum, amniotic fluid, fetal liver, and fetal brain. J Pineal Res 43:74–79
Acknowledgments
This study was supported by a grant from the National Institutes of Health (AG 18884) and Western University of Health Sciences faculty development funds.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Campbell, A., Sharman, E. & Bondy, S.C. Age-related differences in the response of the brain to dietary melatonin. AGE 36, 49–55 (2014). https://doi.org/10.1007/s11357-013-9542-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-013-9542-y