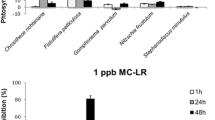
Abstract
Toxins produced by cyanobacteria in freshwater ecosystems constitute a serious health risk worldwide for humans that may use the affected water bodies for recreation, drinking water, and/or irrigation. Cyanotoxins have also been deemed responsible for loss of animal life in many places around the world. This paper explores the effect of H2O2 treatments on cyanobacteria and microcystins in natural samples from a hypertrophic reservoir in microcosm experiments. According to the results, cyanobacteria were more easily affected by H2O2 than by other phytoplanktonic groups. This was shown by the increase in the fractions of chlorophyll-a (a proxy for phytoplankton) and chlorophyll-b (a proxy for green algae) over total phytoplankton pigments and the decrease in the fraction of phycocyanin (a proxy for cyanobacteria) over total phytoplankton pigments. Thus, while an overall increase in phytoplankton occurred, a preferential decrease in cyanobacteria was observed with H2O2 treatments over a few hours. Moreover, significant degradation of total microcystins was observed under H2O2 treatments, while more microcystins were degraded when UV radiation was used in combination with H2O2. The combination of H2O2 and ultraviolet (UV) treatment in natural samples resulted in total microcystin concentrations that were below the World Health Organization limit for safe consumption of drinking water of 1 μg/L. Although further investigation into the effects of H2O2 addition on ecosystem function must be performed, our results show that the application of H2O2 could be a promising method for the degradation of microcystins in reservoirs and the reduction of public health risks related to the occurrence of harmful algal blooms.







Similar content being viewed by others
References
Allen AO, Hochanadel CJ, Ghormley JA, Davis RW (1952) Decomposition of water and aqueous solutions under mixed fast neutron and gamma radiation. J Phys Chem 56(5):575–586
Ananiadis CI (1956) Limnological study of Lake Karla. Bulletin Del’ Institut Oceanographique 1083:1–19
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
APHA (2005) Standard methods for the examination of water and wastewater 21st edition. Published jointly by American Public Health Association, American Water Works Association, and Water Environment Federation
Asada K (2000) The water-water cycle as alternative photon and electron sinks. Philos Trans R Soc Lond Ser B Biol Sci 355:1419–1431
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141:391–396
Barrington DJ, Ghadouani A (2008) Application of hydrogen peroxide for the removal of toxic cyanobacteria and other phytoplankton from wastewater. Envir Sci Tech 42(23):8916–8921
Barrington DJ, Reichwaldt SE, Ghadouani A (2013) The use of hydrogen peroxide to remove cyanobacteria and microcystins from waste stabilization ponds and hypereutrophic systems. Ecol Eng 50:86–94
Bastien C, Cardin R, Veilleux É, Deblois C, Warren A, Laurion I (2011) Performance evaluation of phycocyanin probes for the monitoring of cyanobacteria. Journal of Environmental Monitoring: JEM 13:11–118
Bauzá L, Aguilera A, Ricardo E, Andrinolo D, Giannuzzi L (2014) Application of hydrogen peroxide to the control of eutrophic lake systems in laboratory assays. Toxins (Basel) 6(9):2657–2675
Bittencourt-Oliveira MC, Piccin-Santos V, Moura NA, Aragao-Tavares N, Cordeiro-Araujo M (2014) Cyanobacteria, microcystins and cylindrospermopsin in public drinking supply reservoirs of Brazil. An Acad Bras Ciênc 86(1):297–309
Bolton JR, Linden KG (2003) Standardization of methods for fluence (UV dose) determination in bench-scale UV experiments. J Environ Eng 129(3):209–215
Botes DP, Wessels PL, Kruger H, Runnegar MTC, Santikarn S, Smith RJ, Barna JCJ, Williams DHJ (1985) Structural studies on cyanoginosins-LR, -YR, -YA, and -YM, peptide toxins from Microcystis aeruginosa. J Chem Soc Perkin Trans 1:2747–2448
Briand JF, Jacquet S, Bernard C, Humbert JF (2003) Health hazards for terrestrial vertebrates from toxic cyanobacteria in surface water ecosystems. Vet Res 34:361–377
Chen XC, He SB, Huang YY, Kong HN, Lin Y, Li CJ, Zeng GQ (2009) Laboratory investigation of reducing two algae from eutrophic water treated with light-shading plus aeration. Chemosphere 76:1303–1307
Chorus I (2012) Current approaches to cyanotoxin risk assessment, risk management and regulations in different countries. Umweltbundesamt, Dessau-Rosslau, pp. 71–78
Cook CM, Vardaka E, Lanaras T (2004) Toxic cyanobacteria in Greek freshwaters, 1987–2000: occurrence, toxicity, and impacts in the Mediterranean region. Acta Hydrochim Hydrobiol 32(2):107–124
de la Cruz AA, Antoniou GM, Hiskia A, Pelaez M, Song W, O’Shea EK, He X, Dionysiou DD (2011) Can we effectively degrade microcystins?—implications on human health. Anti Cancer Agents Med Chem 11:19–37
Ding J, Shi H, Timmons T, Adams C (2010) Release and removal of microcystins from Microcystis during oxidative-, physical-, and UV-based disinfection. J Environ Eng 136:2–11
Drabkova M, Admiraal W, Marsalek B (2007) Combined exposure to hydrogen peroxide and light: selective effects on cyanobacteria, green algae, and diatoms. Environ Sci Technol 41(1):309–314
Edwards C, Graham D, Fowler N, Lawton LA (2008) Biodegradation of microcystins and nodularin in freshwaters. Chemosphere 73:1315–1321
Fan J, Hobson P, Lionel H, Daly R, Brookes J (2014) The effects of various control and water treatment processes on the membrane integrity and toxin fate of cyanobacteria. J of Hazard Mater 264:313–322
Fogg GE, Stewart WDP, Fay P, Walsby AE (1973) The bluegreen algae. Academic Press Inc. London, p. 459
Funari E, Testai E (2008) Human health risk assessment related to cyanotoxins exposure. Crit Rev Toxicol 38:97–125
Gao L, Pan X, Zhang D, Mu S, Lee DJ, Halik U (2015) Extracellular polymeric substances buffer against the biocidal effect of H2O2 on the bloom forming cyanobacterium Microcystis aeruginosa. Water Res 69:51–58
Gialis S, Laspidou C (2014) Lake Karla and the contradictory character of Greek environmental policies: a brief historical overview. Proceedings to the IWA regional symposium on water. Wastewater and Environment: Traditions and Culture, Patras
Glaze WH, Kang JW, Chapin DH (1987) The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone Sci Eng 9:335–352
He X, Pelaez M, Westrick JA, O’Shea KE, Hiskia A, Triantis T, Kaloudis T, Stefan MI, de la Cruz AA, Dionysiou DD (2012) Efficient removal of microcystin-LR by UV-C/H2O2 in synthetic and natural water samples. Water Res 46(5):1501–1510
He X, de la Cruz AA, Hiskia A, Kaloudis T, O’Shea K, Dionysiou DD (2015) Destruction of microcystins (cyanotoxins) by UV-254 nm-based direct photolysis and advanced oxidation processes (AOPs): influence of variable amino acids on the degradation kinetics and reaction mechanisms. Water Res 74:227–238
Helman Y, Barkan E, Eisenstadt D, Luz B, Kaplan A (2005) Fractionation of the three stable oxygen isotopes by oxygen-producing and oxygen-consuming reactions in photosynthetic organisms. Plant Physiol 138:2292–2298
Helman Y, Tchernov D, Reinhold L, Shibata M, Ogawa T, Schwarz R, et al. (2003) Genes encoding a-type flavoproteins are essential for photoreduction of O-2 in cyanobacteria. Curr Biol 13:230–235
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equation for determining chlorophyll a, b, c1 and c2. Biochem Physiol Pflanz 167:194–204
Jensen JP, Jeppesen E, Olrik K, Kristensen P (1994) Impact of nutrients and physical factors on the shift from cyanobacterial to chlorophyte dominance in shallow Danish lakes. Can J Fish Aquat Sci 51:1692–1699
Johnk KD, Huisman J, Sharples J, Sommeijeri B, Visser PM, Strooms JM (2008) Summer heat waves promote blooms of harmful cyanobacteria. Glob Change Biol 14:495–512
Kasinak J-ME, Holt BM, Chislock MF, Wilson AE (2015) Benchtop fluorometry of phycocyanin as a rapid approach for estimating cyanobacterial biovolume. J Plankton Res 37(1):248–257
Kull TPJ, Backlund PH, Karlsoon KM, Meriluoto JAO (2004) Oxidation of the cyanobacterial hepatotoxin microcystin-LR by chlorine dioxide: reaction kinetics, characterization, and toxicity of reaction products. Environ Sci Technol 38:6025–6031
Kurmayer R (2011) The toxic cyanobacterium Nostoc sp. strain 152 produces highest amounts of microcystin and nostophycin under stress conditions. J Phycol 47:200–207
Latifi A, Ruiz M, Zhang CC (2009) Oxidative stress in cyanobacteria. FEMS Microbiol Rev 33:258–278
MacElhiney J, Lawton L, Leifert C (2001) Investigations into the inhibitory effects of microcystins on plant growth and the toxicity of plant tissues following exposure. Toxicon 39:1411–1420
Matthijs HC, Visser PM, Reeze B, Meeuse J, Slot PC, Wijn G, Talens R, Huisman J (2012) Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Res 46:1460–1472
Mellios N, Kofinas D, Laspidou C, Papadimitriou T (2015) Mathematical modeling of trophic state and nutrient flows of Lake Karla using the PCLake model. Environ Process 2(1):85–100
Mehler AH (1951) Studies on reactions of illuminated chloroplasts: I. Mechanism of the reduction of oxygen and other hill reagents Arch Biochem Biophys 33:65–77
Moldaenke C (2009) Online fluorescence measurements for monitoring of raw waters and drinking water treatment processes from algae (cyanobacteria) like waters; bbe Moldaenke GmbH: Kronshagen, Germany
Oikonomou A, Κatsiapi M, Karayanni H, Moustaka-Gouni M, Kormas K (2012) Plankton microorganisms coinciding with two consecutive mass fish kills in a newly reconstructed lake. Sci World J. doi:10.1100/2012/504135
Paerl HW, Tucker J, Bland PT (1983) Carotenoid enhancement and its role in maintaining blue-green algal (Microcystis aeruginosa) surface blooms. Limnol Oceanogr 28:847–857
Paerl HW, Huisman J (2008) Blooms like it hot. Science 320:57–58
Papadimitriou Th, Katsiapi M, Kormas ARK, Moustaka-Gouni M, Kagalou I (2013) Artificially-born “killer” lake: phytoplankton based water quality and microcystin affected fish in a reconstructed lake. Sci Total Environ (452–453): 116–124
Passardi F, Zamocky M, Favet J, Jakopitsch C, Penel C, Obinger C, Dunand C (2007) Phylogenetic distribution of catalase-peroxidases: are there patches of order in chaos? Gene 397(1–2):101–113
Patel A, Mishra S, Ghosh PK (2006) Antioxidant potential of C-phycocyanin isolated from cyanobacterial species, Lyngbya, Phormidium and Spirulina species. IJBB 43:25–31
Paul VJ (2008) Global warming and cyanobacterial harmful algal booms. In: Hudnell KH (Ed), Cyanobacterial Harmful Algal Blooms: State of the Science Research Needs Series: Advances in Experimental Medicine and Biology, vol. 619. XXIV, pp. 950
Pearl HW, Hall NS, Calandrino ES (2011) Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci Total Environ 409(10):1739–1745
Posch T, Koster OM, Salcher M, Pernthaler J (2012) Harmful filamentous cyanobacteria favoured by reduced water turnover with lake warming. Nat Clim Chang 2:809–813
Puerto M, Pichardo S, Jos A, Camean AM (2009) Comparison of the toxicity induced by microcystin-RR and microcystin-YR in differentiated and undifferentiated Caco-2 cells. Toxicon 54:161–169
Qian H, Yu S, Sun Z, Xie X, Liu W, Fu Z (2010) Effects of copper sulfate, hydrogen peroxide and N-phenyl-2-napthylamine on oxidative stress and the expression of genes involved photosynthesis and microcystin disposition in Microcystis aeruginosa. Aquat Toxicol 99:405–412
Qian H, Pan X, Chen J, Zhou D, Chen Z, Zhang L, Fu Z (2012) Analyses of gene expression and physiological changes in Microcystis aeruginosa reveal the phytotoxicities of three environmental pollutants. Ecotoxicology 21(3):847–859
Qiao RP, Li N, Qi XH, Wang QS, Zhuang YY (2005) Degradation of microcystin-RR by UV radiation in the presence of hydrogen peroxide. Toxicon 45:745–752
Rapala J, Berg KA, Lyra C, Niemi RM, Manz W, Suomalainen S, Paulin L, Latí K (2005) Paucibacter toxinivorans gen. nov., sp. nov., a bacterium that degrades cyclic cyanobacterial hepatotoxins microcystins and nodularin. Int J Syst Evolut Microbiol 55(4):1563–1568
Reichwaldt ES, Zheng L, Barrington DJ, Ghadouani A (2012) Acute toxicological response of Daphnia and Moina to hydrogen peroxide. J Environ Eng 138:607–611
Rowan KS (1989) Photosynthetic pigments of algae. Cambridge University Press, Cambridge 334 pp
Samuilov VD, Timofeev KN, Sinitsyn SV, Bezryadnov DV (2004) H2O2-induced inhibition of photosynthetic O2 evolution by Anabaena variabilis cells. Biochem Mosc 69(8):926–933
Sarada R, Pillai MG, Ravishankar GA (1999) Phycocyanin from Spirulina sp.: influence of processing of biomass on phycocyanin yield, analysis of efficacy of extraction methods and stability studies on phycocyanin. Process Biochem 34:795–801
Sharma VK, Triantis TM, Antoniou MG, He X, Pelaez M, Han C, Song W, O’Shea KE, de la Cruz AA, Kaloudis T, Hiskia A, Dionysiou DD (2012) Destruction of microcystins by conventional and advanced oxidation processes: a review. Sep Purif Technol 91:3–17
Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, et al. (2002) Regulation and function of ascorbate peroxidase isoenzymes. J ExpBot 53:1305–1319
Shoaf WT, Lium BW (1976) Improved extraction of chlorophyll a and b from algae using dimethyl sulfoxide. Limnol Oceanogr 21:926–928
Stampouli Z, Papadimitriou T, Kagalou I (2012) Evaluation of the ecological state of the ‘new’ Lake Karla (Thessaly) with emphasis on the zooplankton community. Proceedings of the 34th Scientific Conference of Hellenic Association for Biological Sciences, Trikala, May 17–19
Teneva I, Mladenov R, Popov N, Dzhambazov B (2005) Cytotoxicity and apoptotic effects of microcystin-LR and anatoxin-a in mouse lymphocytes. Folia Biologica (Prague) 51(3):62–67
Wada N, Sakamoto T, Matsugo S (2013) Multiple roles of photosynthetic and sunscreen pigments in cyanobacteria focusing on the oxidative stress. Metabolites 3(2):463–483
Wang B, Wang J, Zhang W, Meldrum DR (2012) Application of synthetic biology in cyanobacteria and algae. Front Microbiol 3:344
Wang B, Wang X, Hub Y, Chang M, Bi Y, Hu Z (2015) The combined effects of UV-C radiation and H2O2 on Microcystis aeruginosa, a bloom-forming cyanobacterium. Chemosphere 14:34–43
Ward CJ, Beattie KA, Lee EY, Codd GA (1997) Colorimetric protein phosphatase inhibition assay of laboratory strains and natural blooms of cyanobacteria: comparisons with high-performance liquid chromatographic analysis for microcystins. FEMS Microbiol Lett 153(2):465–473
Weenink EFJ, Luimstra VM (2015) Schuurmans JM. Van Herk MJ, Visser PM and Matthijs HCP
Wetzel RG (2001) Limnology lake and river ecosystems. Academic Press, California
W.H.O. (1998) Cyanobacterial toxins: microcystin-LR. In: Guidelines for drinking water quality. 2nd Edition, Addendum to Vol. 2. Health criteria and other supporting information. World Health Organization, Geneva, Switzerland pp. 95–110
W.H.O. (2003) Algae and cyanobacteria in fresh water. In: Guidelines for safe recreational water environments. Vol. 1: Coastal and fresh waters. World Health Organization, Geneva, Switzerland pp. 136–158
Acknowledgments
This study was supported by the research program LAKEREMAKE, which is implemented under the “ARISTEIA II” Action of the Operational Program “Education and Lifelong Learning” and is co-funded by the European Social Fund (ESF) and National Resources through the National Strategic Reference Framework (NSRF).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Papadimitriou, T., Kormas, K., Dionysiou, D.D. et al. Using H2O2 treatments for the degradation of cyanobacteria and microcystins in a shallow hypertrophic reservoir. Environ Sci Pollut Res 23, 21523–21535 (2016). https://doi.org/10.1007/s11356-016-7418-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7418-2