Abstract
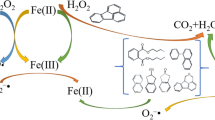
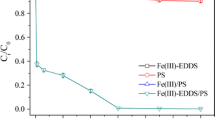
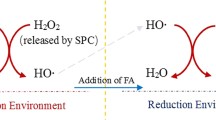
Effective degradation of benzene was achieved in sodium percarbonate (SPC)/Fe(II)-Glu system. The presence of glutamate (Glu) could enhance the regeneration of Fe(III) to Fe(II), which ensures the benzene degradation efficiency at wider pH range and eliminate the influence of HCO3 − in low concentration. Meanwhile, the significant scavenging effects of high HCO3 − concentration could also be overcome by increasing the Glu/SPC/Fe(II)/benzene molar ratio. Free radical probe compound tests, free radical scavenger tests, and electron paramagnetic resonance (EPR) analysis were conducted to explore the reaction mechanism for benzene degradation, in which hydroxyl radical (HO•) and superoxide anion radical (O2 •−) were confirmed as the predominant species responsible for benzene degradation. In addition, the results obtained in actual groundwater test strongly indicated that SPC/Fe(II)-Glu system is applicable for the remediation of benzene-contaminated groundwater in practice.









Similar content being viewed by others
References
Bielski BH, Cabelli DE, Arudi RL, Ross AB (1985) Reactivity of HO2/O2 − radicals in aqueous solution. J Phys Chem Ref Data 14(4):1041–1100
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O−) in aqueous solution. J Phys Chem Ref Data 17(2):513–886
Chang YY, Roh H, Yang JK (2013) Improving the clean-up efficiency of field soil contaminated with diesel oil by the application of stabilizers. Environ Technol 34(11):1481–1487
Chen R, Pignatello JJ (1997) Role of quinone intermediates as electron shuttles in Fenton and photoassisted Fenton oxidations of aromatic compounds. Environ Sci Technol 31(8):2399–2406
Chen G, Hoag GE, Chedda P, Nadim F, Woody BA, Dobbs GM (2001) The mechanism and applicability of in situ oxidation of trichloroethylene with Fenton’s reagent. J Hazard Mater 87(1):171–186
Do SH, Kwon YJ, Kong SH (2011) Feasibility study on an oxidant-injected permeable reactive barrier to treat BTEX contamination: adsorptive and catalytic characteristics of waste-reclaimed adsorbent. J Hazard Mater 191:19–25
Elshafei GM, Yehia F, Dimitry O, Badawi A, Eshaq G (2010) Degradation of nitrobenzene at near neutral pH using Fe2+-glutamate complex as a homogeneous Fenton catalyst. Appl Catal B Environ 99(1):242–247
Fu X, Gu X, Lu S, Miao Z, Xu M, Zhang X, Qiu Z, Sui Q (2015) Benzene depletion by Fe2+-catalyzed sodium percarbonate in aqueous solution. Chem Eng J 267:25–33
Fukuchi S, Nishimoto R, Fukushima M, Zhu Q (2014) Effects of reducing agents on the degradation of 2, 4, 6-tribromophenol in a heterogeneous Fenton-like system with an iron-loaded natural zeolite. Appl Catal B Environ 147:411–419
Gan S, Ng HK (2012) Inorganic chelated modified-Fenton treatment of polycyclic aromatic hydrocarbon (PAH)-contaminated soils. Chem Eng J 180:1–8
Georgi A, Schierz A, Trommler U, Horwitz CP, Collins TJ, Kopinke F-D (2007) Humic acid modified Fenton reagent for enhancement of the working pH range. Appl Catal B Environ 72(1):26–36
Goi A, Viisimaa M, Trapido M, Munter R (2011) Polychlorinated biphenyls-containing electrical insulating oil contaminated soil treatment with calcium and magnesium peroxides. Chemosphere 82(8):1196–1201
Gu X, Lu S, Li L, Qiu Z, Sui Q, Lin K, Luo Q (2011) Oxidation of 1,1,1-trichloroethane stimulated by thermally activated persulfate. Ind Eng Chem Res 50(19):11029–11036
Khan NE, Adewuyi YG (2010) Absorption and oxidation of nitric oxide (NO) by aqueous solutions of sodium persulfate in a bubble column reactor. Ind Eng Chem Res 49(18):8749–8760
Lee Y, Lee W (2010) Degradation of trichloroethylene by Fe(II) chelated with cross-linked chitosan in a modified Fenton reaction. J Hazard Mater 178(1):187–193
Lemaire J, Croze V, Maier J, Simonnot M-O (2011) Is it possible to remediate a BTEX contaminated chalky aquifer by in situ chemical oxidation? Chemosphere 84(9):1181–1187
Lewis S, Lynch A, Bachas L, Hampson S, Ormsbee L, Bhattacharyya D (2009) Chelate-modified Fenton reaction for the degradation of trichloroethylene in aqueous and two-phase systems. Environ Eng Sci 26(4):849–859
Li YC, Bachas LG, Bhattacharyya D (2005) Kinetics studies of trichlorophenol destruction by chelate-based Fenton reaction. Environ Eng Sci 22(6):756–771
Liang CJ, Bruell CJ, Marley MC, Sperry K (2004) Persulfate oxidation for in situ remediation of TCE. I. Activated by ferrous ion with and without a persulfate-thiosulfate redox couple. Chemosphere 55(9):1213–1223
Liang CJ, Wang ZS, Mohanty N (2006) Influences of carbonate and chloride ions on persulfate oxidation of trichloroethylene at 20°C. Sci Total Environ 370(2):271–277
Liang SH, Kao CM, Kuo YC, Chen KF (2011) Application of persulfate-releasing barrier to remediate MTBE and benzene contaminated groundwater. J Hazard Mater 185(2):1162–1168
Lipczynska-Kochany E, Kochany J (2008) Effect of humic substances on the Fenton treatment of wastewater at acidic and neutral pH. Chemosphere 73(5):745–750
Ma JJ, Ma WH, Song WJ, Chen CC, Tang YL, Zhao JC, Huang YP, Xu YM, Zang L (2006) Fenton degradation of organic pollutants in the presence of low-molecular-weight organic acids: cooperative effect of quinone and visible light. Environ Sci Technol 40(2):618–624
Miao Z, Gu X, Lu S, Zang X, Wu X, Xu M, Ndong LBB, Qiu Z, Sui Q, Fu GY (2015) Perchloroethylene (PCE) oxidation by percarbonate in Fe2+-catalyzed aqueous solution: PCE performance and its removal mechanism. Chemosphere 119:1120–1125
Monteagudo J, Durán A, Martín IS, Aguirre M (2010) Effect of light source on the catalytic degradation of protocatechuic acid in a ferrioxalate-assisted photo-Fenton process. Appl Catal B Environ 96(3):486–495
Northup A, Cassidy D (2008) Calcium peroxide (CaO2) for use in modified Fenton chemistry. J Hazard Mater 152(3):1164–1170
Park MG, Choi MH (2006) Method for preparation of amino acid chelate. US Patent, U.S., (2006)/0128799AI 2006
Pignatello JJ, Oliveros E, MacKay A (2006) Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit Rev Environ Sci Technol 36(1):1–84
Qian Y, Zhou X, Zhang Y, Zhang W, Chen J (2013) Performance and properties of nanoscale calcium peroxide for toluene removal. Chemosphere 91(5):717–723
Rosen GM, Beselman A, Tsai P, Pou S, Mailer C, Ichikawa K, Robinson BH, Nielsen R, Halpern HJ, MacKerell AD (2004) Influence of conformation on the EPR spectrum of 5, 5-dimethyl-1-hydroperoxy-1-pyrrolidinyloxyl: a spin trapped adduct of superoxide. J Org Chem 69(4):1321–1330
Seol Y, Javandel I (2008) Citric acid-modified Fenton’s reaction for the oxidation of chlorinated ethylenes in soil solution systems. Chemosphere 72(4):537–542
Sun YF, Pignatello JJ (1992) Chemical treatment of pesticide wastes-evaluation of Fe(III) chelates for catalytic hydrogen-peroxide oxidation of 2,4-D at circumneutral pH. J Agric Food Chem 40(2):322–327
Sunder M, Hempel DC (1997) Oxidation of tri- and perchloroethene in aqueous solution with ozone and hydrogen peroxide in a tube reactor. Water Res 31(1):33–40
Tamura H, Goto K, Yotsuyanagi T, Nagayama M (1974) Spectrophotometric determination of iron (II) with 1, 10-phenanthroline in the presence of large amounts of iron (III). Talanta 21(4):314–318
Teel AL, Watts RJ (2002) Degradation of carbon tetrachloride by modified Fenton’s reagent. J Hazard Mater 94(2):179–189
Valentine RL, Wang HA (1998) Iron oxide surface catalyzed oxidation of quinoline by hydrogen peroxide. J Environ Eng 124(1):31–38
Walling C, Goosen A (1973) Mechanism of the ferric ion catalyzed decomposition of hydrogen peroxide. Effect of organic substrates. J Am Chem Soc 95(9):2987–2991
Watts RJ, Haller DR, Jones AP, Teel AL (2000) A foundation for the risk-based treatment of gasoline-contaminated soils using modified Fenton’s reactions. J Hazard Mater 76(1):73–89
Yang GC, Liu CY (2001) Remediation of TCE contaminated soils by in situ EK-Fenton process. J Hazard Mater 85(3):317–331
Yap CL, Gan S, Ng HK (2011) Fenton based remediation of polycyclic aromatic hydrocarbons-contaminated soils. Chemosphere 83(11):1414–1430
Zang X, Gu X, Lu S, Qiu Z, Sui Q, Lin K, Du X (2014) Trichloroethylene oxidation performance in sodium percarbonate (SPC)/Fe2+ system. Environ Technol 35(7):791–798
Acknowledgments
This study was financially supported by the grant from the National Natural Science Foundation of China (No. 41373094 and No. 51208199), China Postdoctoral Science Foundation (2015M570341), and the Fundamental Research Funds for the Central Universities (22A201514057 and 222201514339).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Santiago V. Luis
Xiaogang Gu contributed significantly to this paper.
Rights and permissions
About this article
Cite this article
Fu, X., Gu, X., Lu, S. et al. Enhanced degradation of benzene by percarbonate activated with Fe(II)-glutamate complex. Environ Sci Pollut Res 23, 6758–6766 (2016). https://doi.org/10.1007/s11356-015-5908-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5908-2