Abstract
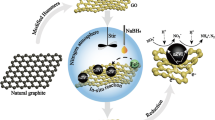
Graphene oxide (GO) and nano-sized zero-valent iron-reduced graphene oxide (nZVI-rGO) composite were prepared. The GO and nZVI-rGO composite were characterized by transmission electron microscopy (TEM), Fourier transform infrared (FTIR), energy-dispersive spectroscopy (EDS), and Raman spectroscopy. The size of nZVI was about 6 nm as observed by TEM. The system of nZVI-rGO and persulfate (PS) was used for the degradation of trichloroethylene (TCE) in water, and showed 26.5 % more efficiency as compared to nZVI/PS system. The different parameters were studied to determine the efficiency of nZVI-rGO to activate the PS system for the TCE degradation. By increasing the PS amount, TCE removal was also improved while no obvious effect was observed by varying the catalyst loading. Degradation was decreased as the TCE initial concentration was increased from 20 to 100 mg/L. Moreover, when initial solution pH was increased, efficiency deteriorated to 80 %. Bicarbonate showed more negative effect on TCE removal among the solution matrix. To better understand the effects of radical species in the system, the scavenger tests were performed. The •SO4 − and •O2 − were predominant species responsible for TCE removal. The nZVI-rGO-activated PS process shows potential applications in remediation of highly toxic organic contaminants such as TCE present in the groundwater.

Persulfate activated by reduced graphene oxide and nano-sized zero-valent iron composite can be used for efficient degradation of trichloroethylene (TCE) in water.









Similar content being viewed by others
References
Adewuyi YG, Sakyi NY (2013) Removal of nitric oxide by aqueous sodium persulfate simultaneously activated by temperature and Fe2+ in a lab-scale bubble reactor. Ind Eng Chem Res 52:14687–14697
Al-Shamsi MA, Thomson NR (2013) Treatment of organic compounds by activated persulfate using nanoscale zerovalent iron. Ind Eng Chem Res 52:13564–13571
Andreozzi R, Caprio V, Insola A, Marotta R (1999) Advanced oxidation processes (AOP) for water purification and recovery. Catal Today 53:51–59
Anipsitakis GP, Dionysiou DD (2004a) Radical generation by the interaction of transition metals with common oxidants. Environ Sci Technol 38:3705–3712
Anipsitakis GP, Dionysiou DD (2004b) Transition metal/UV-based advanced oxidation technologies for water decontamination. Appl Catal B Environ 54:155–163
Anipsitakis GP, Dionysiou DD, Gonzalez MA (2006) Cobalt-mediated activation of peroxymonosulfate and sulfate radical attack on phenolic compounds. Implications of chloride ions. Environ Sci Technol 40:1000–1007
Cheng P, Yang Z, Wang H, Cheng W, Chen M, Shangguan W, Ding G (2012) TiO2–graphene nanocomposites for photocatalytic hydrogen production from splitting water. Int J Hydrog Energy 37:2224–2230
Chiu WA, Jinot J, Scott CS, Makris SL, Cooper GS, Dzubow RC, Bale AS, Evans MV, Guyton KZ, Keshava N (2013) Human health effects of trichloroethylene: key findings and scientific issues. Environ Health Perspect 121:303–311
Fang GD, Dionysiou DD, Wang Y, Al-Abed SR, Zhou D-M (2012) Sulfate radical-based degradation of polychlorinated biphenyls: effects of chloride ion and reaction kinetics. J Hazard Mater 227:394–401
Fang GD, Dionysiou DD, Al-Abed SR, Zhou D-M (2013) Superoxide radical driving the activation of persulfate by magnetite nanoparticles: implications for the degradation of PCBs. Appl Catal B Environ 129:325–332
Furman O, Laine DF, Blumenfeld A, Teel AL, Shimizu K, Cheng IF, Watts RJ (2009) Enhanced reactivity of superoxide in water–solid matrices. Environ Sci Technol 43:1528–1533
Furukawa Y, Kim JW, Watkins J, Wilkin RT (2002) Formation of ferrihydrite and associated iron corrosion products in permeable reactive barriers of zero-valent iron. Environ Sci Technol 36:5469–5475
Gao YQ, Gao NY, Deng Y, Yang YQ, Ma Y (2012) Ultraviolet (UV) light-activated persulfate oxidation of sulfamethazine in water. Chem Eng J 195–296:248–253
Gomathi Devi L, Girish Kumar S, Mohan Reddy K, Munikrishnappa C (2009) Photo degradation of methyl orange an azo dye by advanced fenton process using zero valent metallic iron: influence of various reaction parameters and its degradation mechanism. J Hazard Mater 164:459–467
Guo J, Wang R, Tjiu WW, Pan J, Liu T (2012) Synthesis of Fe nanoparticles@graphene composites for environmental applications. J Hazard Mater 225–226:63–73
Gupta VK, Atar N, Yola ML, Üstündağ Z, Uzun L (2014) A novel magnetic Fe@Au core–shell nanoparticles anchored graphene oxide recyclable nanocatalyst for the reduction of nitrophenol compounds. Water Res 48:210–217
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Hussain I, Zhang Y, Huang S (2014) Degradation of aniline with zero-valent iron as an activator of persulfate in aqueous solution. RSC Adv 4:3502–3511
Jabeen H, Chandra V, Jung S, Lee JW, Kim KS, Kim SB (2011) Enhanced Cr(vi) removal using iron nanoparticle decorated graphene. Nanoscale 3:3583–3585
Jabeen H, Kemp KC, Chandra V (2013) Synthesis of nano zerovalent iron nanoparticles—graphene composite for the treatment of lead contaminated water. J Environ Manag 130:429–435
Kim H-S, Ahn JY, Kim C, Lee S, Hwang I (2014) Effect of anions and humic acid on the performance of nanoscale zero-valent iron particles coated with polyacrylic acid. Chemosphere 113:93–100
Ko S, Crimi M, Marvin BK, Holmes V, Huling SG (2012) Comparative study on oxidative treatments of NAPL containing chlorinated ethanes and ethenes using hydrogen peroxide and persulfate in soils. J Environ Manag 108:42–48
Kube M, Beck A, Zinder SH, Kuhl H, Reinhardt R, Adrian L (2005) Genome sequence of the chlorinated compound-respiring bacterium Dehalococcoides species strain CBDB1. Nat Biotechnol 23:1269–1273
Lai B, Chen Z, Zhou Y, Yang P, Wang J, Chen Z (2013) Removal of high concentration p-nitrophenol in aqueous solution by zero valent iron with ultrasonic irradiation (US–ZVI). J Hazard Mater 250–251:220–228
Li H, Wan J, Ma Y, Huang M, Wang Y, Chen Y (2014) New insights into the role of zero-valent iron surface oxidation layers in persulfate oxidation of dibutyl phthalate solutions. Chem Eng J 250:137–147
Li R, Jin X, Megharaj M, Naidu R, Chen Z (2015a) Heterogeneous Fenton oxidation of 2,4-dichlorophenol using iron-based nanoparticles and persulfate system. Chem Eng J 264:587–594
Li Z, Chen Z, Xiang Y, Ling L, Fang J, Shang C, Dionysiou DD (2015b) Bromate formation in bromide-containing water through the cobalt-mediated activation of peroxymonosulfate. Water Res. doi:10.1016/j.watres.2015.06.019
Liang C, Guo YY (2010) Mass transfer and chemical oxidation of naphthalene particles with zerovalent iron activated persulfate. Environ Sci Technol 44:8203–8208
Liang C, Lai MC (2008) Trichloroethylene degradation by zero valent iron activated persulfate oxidation. Environ Eng Sci 25:1071–1077
Liang C, Su HW (2009) Identification of sulfate and hydroxyl radicals in thermally activated persulfate. Ind Eng Chem Res 48:5558–5562
Liang C, Bruell CJ, Marley MC, Sperry KL (2004) Persulfate oxidation for in situ remediation of TCE. II. Activated by chelated ferrous ion. Chemosphere 55:1225–1233
Liang C, Wang ZS, Mohanty N (2006) Influences of carbonate and chloride ions on persulfate oxidation of trichloroethylene at 20C. Sci Total Environ 370:271–277
Liang C, Wang ZS, Bruell CJ (2007) Influence of pH on persulfate oxidation of TCE at ambient temperatures. Chemosphere 66:106–113
Liang C, Huang CF, Chen YJ (2008a) Potential for activated persulfate degradation of BTEX contamination. Water Res 42:4091–4100
Liang C, Huang CF, Mohanty N, Kurakalva RM (2008b) A rapid spectrophotometric determination of persulfate anion in ISCO. Chemosphere 73:1540–1543
Liu CS, Shih K, Sun CX, Wang F (2012) Oxidative degradation of propachlor by ferrous and copper ion activated persulfate. Sci Total Environ 416:507–512
Liu F, Yang J, Zuo J, Ma D, Gan L, Xie B, Wang P, Yang B (2014) Graphene-supported nanoscale zero-valent iron: removal of phosphorus from aqueous solution and mechanistic study. J Environ Sci 26:1751–1762
Lou X, Wu L, Guo Y, Chen C, Wang Z, Xiao D, Fang C, Liu J, Zhao J, Lu S (2014) Peroxymonosulfate activation by phosphate anion for organics degradation in water. Chemosphere 117:582–585
Oh SY, Kim HW, Park JM, Park HS, Yoon C (2009) Oxidation of polyvinyl alcohol by persulfate activated with heat, Fe2+, and zero-valent iron. J Hazard Mater 168:346–351
Phenrat T, Saleh N, Sirk K, Tilton RD, Lowry GV (2006) Aggregation and sedimentation of aqueous nanoscale zerovalent iron dispersions. Environ Sci Technol 41:284–290
Roshani B, Karpel Vel Leitner N (2011) Effect of persulfate on the oxidation of benzotriazole and humic acid by e-beam irradiation. J Hazard Mater 190:403–408
Sun Y, Ding C, Cheng W, Wang X (2014) Simultaneous adsorption and reduction of U(VI) on reduced graphene oxide-supported nanoscale zerovalent iron. J Hazard Mater 280:399–408
Tamura H, Goto K, Yotsuyanagi T, Nagayama M (1974) Spectrophotometric determination of iron(II) with 1,10-phenanthroline in the presence of large amounts of iron(III). Talanta 21:314–318
Tan C, Gao N, Chu W, Li C, Templeton MR (2012) Degradation of diuron by persulfate activated with ferrous ion. Sep Purif Technol 95:44–48
Teel AL, Watts RJ (2002) Degradation of carbon tetrachloride by modified Fenton’s reagent. J Hazard Mater 94:179–189
Thompson RC (1981) Catalytic decomposition of peroxymonosulfate in aqueous perchloric acid by the dual catalysts silver(1+) and peroxydisulfate(2-) and by cobalt(2+). Inorg Chem 20:1005–1010
Usman M, Faure P, Ruby C, Hanna K (2012) Application of magnetite-activated persulfate oxidation for the degradation of PAHs in contaminated soils. Chemosphere 87:234–240
Vikesland PJ, Heathcock AM, Rebodos RL, Makus KE (2007) Particle size and aggregation effects on magnetite reactivity toward carbon tetrachloride. Environ Sci Technol 41:5277–5283
Wang D, Bolton JR, Hofmann R (2012) Medium pressure UV combined with chlorine advanced oxidation for trichloroethylene destruction in a model water. Water Res 46:4677–4686
Wang H, Li S, Si Y, Sun Z, Li S, Lin Y (2014a) Recyclable enzyme mimic of cubic Fe3O4 nanoparticles loaded on graphene oxide-dispersed carbon nanotubes with enhanced peroxidase-like catalysis and electrocatalysis. J Mater Chem B 2:4442–4448
Wang X, Wang L, Li J, Qiu J, Cai C, Zhang H (2014b) Degradation of Acid Orange 7 by persulfate activated with zero valent iron in the presence of ultrasonic irradiation. Sep Purif Technol 122:41–46
Xu HB, Zhao DY, Li YJ, Liu PY, Dong CX (2014a) Enhanced degradation of ortho-nitrochlorobenzene by the combined system of zero-valent iron reduction and persulfate oxidation in soils. Environ Sci Pollut Res 21:5132–5140
Xu M, Du H, Gu X, Lu S, Qiu Z, Sui Q (2014b) Generation and intensity of active oxygen species in thermally activated persulfate systems for the degradation of trichloroethylene. RSC Adv 4:40511–40517
Zhang YQ, Xie XF, Huang SB, Liang HY (2014) Effect of chelating agent on oxidation rate of aniline in ferrous ion activated persulfate system at neutral pH. J Cent South Univ 21:1441–1447
Zhen G, Lu X, Zhao Y, Chai X, Niu D (2012) Enhanced dewaterability of sewage sludge in the presence of Fe(II)-activated persulfate oxidation. Bioresour Technol 116:259–265
Zhou D, Chen L, Zhang C, Yu Y, Zhang L, Wu F (2014) A novel photochemical system o ferrous sulfite complex: kinetics and mechanisms of rapid decolorization of Acid Orange 7 in aqueous solutions. Water Res 57:87–95
Zhou J, Xiao J, Xiao D, Guo Y, Fang C, Lou X, Wang Z, Liu J (2015) Transformations of chloro and nitro groups during the peroxymonosulfate-based oxidation of 4-chloro-2-nitrophenol. Chemosphere 446–451
Zuo Z, Cai Z, Katsumura Y, Chitose N, Muroya Y (1999) Reinvestigation of the acid–base equilibrium of the (bi)carbonate radical and pH dependence of its reactivity with inorganic reactants. Radiat Phys Chem 55:15–23
Acknowledgments
We acknowledge the financial support from the National Natural Science Foundation of China (nos. 51273063, 21476143, and 21306049), the Fundamental Research Funds for the Central Universities, the higher school specialized research fund for the doctoral program (222201313005 and 222201314029), 111 Project Grant (B08021), the Open Project of State Key Laboratory of Chemical Engineering (SKL-ChE-14C01).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Santiago V. Luis
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 34 kb)
Rights and permissions
About this article
Cite this article
Ahmad, A., Gu, X., Li, L. et al. Efficient degradation of trichloroethylene in water using persulfate activated by reduced graphene oxide-iron nanocomposite. Environ Sci Pollut Res 22, 17876–17885 (2015). https://doi.org/10.1007/s11356-015-5034-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5034-1