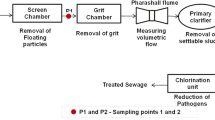
Abstract
A gas chromatography/mass spectrometry (GC/MS)-based method was developed for simultaneous determination of triclosan (TCS) and its degradation products including 2,4-dichlorophenol (2,4-DCP), 2,8-dichlorodibenzo-p-dioxin (2,8-DCDD), and methyl triclosan (MTCS) in wastewater and sludge samples. The method provides satisfactory detection limit, accuracy, precision and recovery especially for samples with complicated matrix such as sewage sludge. Liquid-liquid extraction and accelerated solvent extraction (ASE) methods were applied for the extraction, and column chromatography was employed for the sample cleanup. Analysis was performed by GC/MS in the selected ion monitoring (SIM) mode. The method was successfully applied to wastewater and sludge samples from three different municipal wastewater treatment plants (WWTPs). Satisfactory mean recoveries were obtained as 91(±4)–106(±7) %, 82(±3)–87(±4) %, 86(±6)–87(±8) %, and 88(±4)–105(±3) % in wastewater and 88(±5)–96(±8) %, 84(±2)–87(±3) %, 84(±7)–89(±4) %, and 88(±3)–97(±5) % in sludge samples for TCS, 2,4-DCP, 2,8-DCDD, and MTCS, respectively. TCS degradation products were detected based on the type of the wastewater and sludge treatment. 2,8-DCDD was detected in the plant utilizing UV disinfection at the mean level of 20.3(±4.8) ng/L. 2,4-DCP was identified in chemically enhanced primary treatment (CEPT) applying chlorine disinfection at the mean level of 16.8(±4.5) ng/L). Besides, methyl triclosan (MTCS) was detected in the wastewater collected after biological treatment (10.7 ± 3.3 ng/L) as well as in sludge samples that have undergone aerobic digestion at the mean level of 129.3(±17.2) ng/g dry weight (dw).




Similar content being viewed by others
References
Aranami K, Readman JW (2007) Photolytic degradation of triclosan in freshwater and seawater. Chemosphere 66:1052–1056
Balmer M, Poiger T, Droz C, Romanin K, Bergqvist P, Müller M, Buser H (2004) Occurrence of methyl triclosan, a transformation product of the bactericide triclosan, in fish from various lakes in Switzerland. Environ Sci Technol 38:390–395
Bester K (2005) Fate of triclosan and triclosan-methyl in sewage treatment plants and surface waters. Arch Environ Contam Toxicol 49:9–17
Buth JM, Grandbois M, Vikesland PJ, Mcneill K, Arnold WA (2009) Aquatic photochemistry of chlorinated triclosan derivatives: potential source of polychlorodibenzo-p-dioxins. Environ Toxicol Chem 28:2555–2563
Buth JM, Steen PO, Sueper CH, Blumentritt D, Vikesland PJ, Arnold WA, Neill KM (2010) Dioxin photoproducts of triclosan and its chlorinated derivatives in sediment cores. Environ Sci Technol 44:4545–4551
Butler E, Whelan MJ, Sakrabani R, van Egmond R (2012) Fate of triclosan in field soils receiving sewage sludge. Environ Pollut 167:101–109
Canosa P, Morales S, Rodríguez I, Rubí E, Cela R, Gómez M (2005) Aquatic degradation of triclosan and formation of toxic chlorophenols in presence of low concentrations of free chlorine. Anal Bioanal Chem 383:1119–1126
Chen X, Jeppe L-N, Furgal K, Liu Y, Lolas I-B, Bester K (2011) Biodegradation of triclosan and formation of methyl-triclosan in activated sludge under aerobic conditions. Chemosphere 84(4):452–456
Chow AYK, Hirsch GH, Buttar HS (1977) Nephrotoxic and hepatotoxic effects of triclosan and chlorhexidine in rats. Toxicol Appl Pharmacol 42:1
Colby BN, Rosecrance AE, Colby ME (1981) Measurement parameter selection for quantitative isotope dilution gas chromatography/mass spectrometry. Anal Chem 53:1907–1911
Dai X, Zhao Y, Li M, Fang X, Li X, Li H, Xu B (2012) Determination of urea in milk by liquid chromatography isotope dilution mass spectrometry. Anal Lett 45:1557–1565
Diemer J, Heumann KG (2000) Development of an ICP-IDMS method for accurate routine analyses of toxic heavy metals in polyolefins and comparison with results by TI-IDMS. Fresen J Anal Chem 368:103–108
Federle T, Kaiser S, Nuck B (2002) Fate and effects of triclosan in activated sludge. Environ Toxicol Chem 21:1330–1337
Ferrer I, Mezcua M, Gomez MJ, Thurman EM, Aguera A, Hernando MD, Fernandez-Alba AR (2004) Liquid chormatography/time-of-flight mass spectrometric analysis for the elucidation of the photodegradation products of triclosan in wastewater samples. Rapid Commun Mass Spectrom 18:443–450
Gatidou G, Thomaidis NS, Stasinakis AS, Lekkas TD (2007) Simultaneous determination of the endocrine disrupting compounds nonylphenol, nonylphenol ethoxylates, triclosan and bisphenol A in wastewater and sewage sludge by gas chromatography-mass spectrometry. J Chromatogr A 1138:32–41
Glaze WH, Henderson JE (1975) Formation of organochlorine compounds from the chlorination of a municipal secondary effluent. J Water Pollut Control Fed 47(10):2511–2515
Greyshock AE, Vikesland PJ (2006) Triclosan reactivity in chloraminated waters. Environ Sci Technol 40:2615–2622
International Agency for Research on Cancer, World Health Organization (1999) Hydrazine and hydrogen peroxide summary of data reported and evaluation, re-evaluation of some organic chemicals
Itoh N, Fushimi A, Yarita T, Aoyagi Y, Numata M (2011) Accurate quantification of polycyclic aromatic hydrocarbons in dust samples using microwave-assisted solvent extraction combined with isotope-dilution mass spectrometry. Anal Chim Acta 699:49–56
Jones RD, Jampani HB, Newman JL, Lee AS (2000) Triclosan: a review of effectiveness and safety in health care settings. Am J Infect Control 28:184–196
Kanetoshi A, Ogawa H, Katsura E, Kaneshima H (1987) Chlorination of Irgasan DP3000 and formation of dioxins from its chlorinated derivatives. J Chromatogr A 389:139–153
Kanetoshi A, Ogawa H, Katsura E, Kaneshima H (1988) Formation of polychlorinated dibenzo-p-dioxin from 2,4,4′-trichloro-2′-hydroxydiphenyl ether (Irgasan DP3000) and its chlorinated derivatives by exposure to sunlight. J Chromatogr A 454:145–155
Kazama M, Mizuishi K, Nakamura Y, Harada H, Totani T (1974) Studies on analytical method of specific materials in cosmetics IX analysis of hexachlorophene by thin-layer and gas chromatography. Eisei Kagaku 20:248
Krasner SW, Weinberg HS, Richardson SD, Pastor SJ, Chinn R, Sclimenti MJ, Onstad GD, Thruston AD Jr (2006) Occurrence of a new generation of disinfection by-products. Environ Sci Techmol 40(23):7175–7185
Latch DE, Packer JL, Arnold WA, McNeill K (2003) Photochemical conversion of triclosan to 2,8-dichlorodibenzop-dioxin in aqueous solution. J Photochem Photobiol A Chem 158:63–66
Latch DE, Packer JL, Stender B, Vanoverbeke J, Arnold WA, McNeill K (2005) Aqueous photochemistry of triclosan, formation of 2,4-dichlorophenol, 2,8-dichlorodibenzo-p-dioxin and oligomerization products. Environ Toxicol Chem 24:517–525
Leikera TJ, Abneya SR, Goodbred SL, Rosenc MR (2009) Identification of methyl triclosan and halogenated analogues in male common carp (Cyprinus carpio) from Las Vegas Bay and semipermeable membrane devices from Las Vegas Wash, Nevada. Sci Total Enviorn 407:2102–2114
Li J (2001) Prediction of internal standards in reversed-phase liquid chromatography 1. Initial study on predicting internal standards for use with neutral samples based on linear solvation energy relationships. J Chromatogr A 927:19–30
Lindstorm A, Buerge IJ, Spoiger T, Bergqvist P-A, Muller MD, Rudolfbuser H (2002) Occurrence and environmental behavior of the bactericide triclosan and its methyl derivative in surface waters and in wastewater. Environ Sci Technol 36:2322–2329
Lores M, Llompart M, Sanchez-Prado L, Garcia-Jares C, Cela R (2005) Confirmation of the formation of dichlorodibenzo-p-dioxin in the photodegradation of triclosan by photo-SMPE. Anal Bioanal Chem 381:1294–1298
McAvoy DC, Schatowitz B, Jacob M, Hauk A, Eckhoff WS (2002) Measurement of triclosan in wastewater treatment systems. Environ Toxicol Chem 21:1323–1329
Mechlińska A, Wolska L, Czarzbon I, Namieśnik J (2012) Comparison of different extraction techniques of PCBs from sediment samples using the isotope dilution mass spectrometry technique. Crit Rev Anal Chem 42:184–191
Mezcua M, Gómez MJ, Ferrer I, Aguera A, Hernando MD, Fernández-Alba AR (2004) Evidence of 2,7/2,8-dibenzodichloro-p-dioxin as a photodegradation product of triclosan in water and wastewater samples. Anal Chim Acta 524:241–247
Milton JTM, Wang J (2002) High accuracy method for isotope dilution mass spectrometry with application to the measurement of carbon dioxide. Int J Mass Spectrom 218:63–73
Miyazaki T, Yamagishi T, Matsumoto M (1984) Residues of 4-chloro-1-(2,4-dichlorophenoxy)-2-methoxybenzene(triclosan methyl) in aquatic biota. Bull Environ Contam Toxicol 32:227–232
O’Neil M (2001) The Merck index—an encyclopedia of chemicals, drugs, and biologicals. 13th ed. Whitehouse Station (NJ): Merck and Co., Inc. p. 1447. [cited in HSDB 2007]
Okumura T, Nishikawa Y (1996) Gas chromatography-mass spectrometry determination of triclosan in water, sediment and fish samples via methylation with diazomethane. Anal Chim Acta 325:175–184
Puthiya Veetil PG, Vijaya Nadaraja A, Bhasi A, Khan S, Bhaskaran K (2012) Degradation of triclosan under aerobic, anoxic and anaerobic conditions. Appl Biochem Biotechnol 167:1603–1612
Samara F, Gullett B, Harrison R, Chu A, Clark G (2009) Determination of relative assay response factors for toxic chlorinated and brominated dioxins/furans using an enzyme immunoassay (EIA) and a chemically-activated luciferase gene expression cell bioassay (CALUX). Environ Int 35:588–593
Samaras VG, Thomaidis NS, Stasinakis AS, Lekkas TD (2011) An analytical method for the simultaneous trace determination of acidic pharmaceuticals and phenolic endocrine disrupting chemicals in wastewater and sewage sludge by gas chromatography-mass spectrometry. Anal Bioanal Chem 399:2549–2561
Singer H, Muller S, Tixier C, Pillonel L (2002) Triclosan: occurrence and fate of a widely used biocide in the aquatic environment: field measurements in wastewater treatment, plants, surface waters, and lake sediments. Environ Sci Technol 36:4998–5004
Son H-S, Choi S-B, Zoh K-D, Khan E (2007) Effects of ultraviolet intensity and wavelength on the photolysis of triclosan. Water Sci Technol 55:209–216
Tan LJ, Nielsen N, Young DC, Trizna Z (2002) Use of antimicrobial agents in consumer products. Arch Dermatol 138:1082–1086
US Environmental Protection Agency (1994) Emergency Planning and Community Right to Know Act. http://www.epa.gov/agriculture/lcra.html.
Vogl J, Pritzkow W (2010) Isotope dilution mass spectrometry—a primary method of measurement and its role for RM certification. MAPAN- J Metrol Soc I 25:135–164
Wang Z, Lau BP-Y, Tague B, Sparling M, Forsyth D (2011) Determination of perchlorate in infant formula by isotope dilution ion chromatography/tandem mass spectrometry. Food Addit Contam: Part A 28:799–806
Waria M, O’Connor GA, Toor GS (2011) Biodegradation of triclosan in biosolids-amended soils. Environ Toxicol Chem 30:2488–2496
Ying G, Kookana RS (2007) Triclosan in wastewaters and biosolids from Australian wastewater treatmetn plants. Environ Int 33:199–205
Funding
This study was supported by the National Natural Science Foundation of China (21175025) and Faculty Research Grant from Hong Kong Baptist University (FRG2/12-13/032).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Leif Kronberg
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 34 kb)
Rights and permissions
About this article
Cite this article
Tohidi, F., Cai, Z. GC/MS analysis of triclosan and its degradation by-products in wastewater and sludge samples from different treatments. Environ Sci Pollut Res 22, 11387–11400 (2015). https://doi.org/10.1007/s11356-015-4289-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4289-x