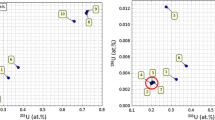
Abstract
Since primary environmental concept for long storage of nuclear waste involved assessment of water in uranium complexes depending on migration processes, the paper emphasized solid-state matrix-assisted laser desorption/ionization (MALDI) mass spectrometric (MS) and IR spectroscopic determination of UO2(NO3)2·6H2O; UO2(NO3)2·3H2O, α-, β-, and γ-UO3 modifications; UO3·xH2O (x = 1 or 2); UO3·H2O, described chemically as UO2(OH)2, β- and γ-UO2(OH)2 modifications; and UO4·2H2O, respectively. Advantages and limitation of vibrational spectroscopic approach are discussed, comparing optical spectroscopic data and crystallographic ones. Structural similarities occurred in α–γ modifications of UO3, and UO2(OH)2 compositions are analyzed. Selective speciation achieved by solid-state mass spectrometry is discussed both in terms of its analytical contribution for environmental quality assurance and assessment of radionuclides, and fundamental methodological interest related the mechanistic complex water exchange of UO3·H2O forms in the gas phase. In addition to high selectivity and precision, UV-MALDI-MS, employing an Orbitrap analyzer, was a method that provided fast steps that limited sample pretreatment techniques for direct analysis including imaging. Therefore, random and systematic errors altering metrology and originating from the sample pretreatment stages in the widely implemented analytical protocols for environmental sampling determination of actinides are significantly reduced involving the UV-MALDI-Orbitrap-MS method. The method of quantum chemistry is utilized as well to predict reliably the thermodynamics and nature of U–O bonds in uranium species in gas and condensed phases.





Similar content being viewed by others
References
Allen G, Tempest P (1986) Ordered defects in the oxides of uranium. Proc R Soc Lond A 406:325–344
Bannister M, Taylor J (1970) The crystal structure and anisotropic thermal expansion of p-uranyl dihydroxide, β-UO2(OH)2. Acta Cryst B26:1775–1781
Beauchamp J (1976) Properties and reactions of uranium hexafluoride by ion cyclotron resonance spectroscopy. J Chem Phys 64:718
Beer S, Berryman O, Ajami D, Rebek J Jr (2010) Encapsulation of the uranyl dication. Chem Sci 1:43–47
Berthet J, Nierlich M, Ephritikhine M (2003) A novel coordination geometry for the uranyl ion. Rhombohedral uranium environment in [UO2(OTf)2(bpy)2] and [UO2(phen)3][OTf]2, Chem Comm 1660–1661
Choi J, Lamshoeft M, Zühlke S, Park K, Shim J, Spiteller M (2012) Determination of sedatives and adrenergic blockers in blood meal using accelerated solvent extraction and Orbitrap mass spectrometry. J Chromatogr A 1260:111–119
Cole R (Ed.) (2010) Electrospra and MALDI mass spectrometry, Wiley, New York, 2nd Ed., pp. 1–613.
Chien W, Anbalagan V, Zandler M, Van Stipdonk M, Hanna D, Gresham G, Groenewold G (2004) Intrinsic hydration of monopositive uranyl hydroxide, nitrate, and acetate cations. J Am Soc Mass Spectrom 15:777–783
Compton R (1977) On the formation of positive and negative ions in gaseous UF6 citation. J Chem Phys 66:4478
Cornett D, Frappier S, Caprioli R (2008) MALDI-FTICR imaging mass spectrometry of drugs and metabolites in tissue. Anal Chem 80:5648–5653
Crawford C, Fugate G, Cable-Dunlap P, Walla N, Siems W, Hilla H (2013) The novel analysis of uranyl compounds by electrospray–ion mobility–mass spectrometry, http://dx.doi.org/10.1016/j.ijms.2012.08.004
Dalton 2011 Program Package; http://www.daltonprogram.org/download.html
Debets P (1966) The Structure of β-UO3. Acta Cryst 21:589–593
Denning R (1992) Electronic structure and bonding in actinyl ions, structure and bondin. Springer, Berlin, pp 215–276
Denning R (2007) Electronic structure and bonding in actinyl ions and their analogs. J Phys Chem A 111:4125–4143
Del Carmen MM, Marcalo J, Russo N, Gibson J (2010) Gas-phase reactions of uranate ions, UO2 −, UO3 −UO4 −, and UO4H−, with methanol: a convergence of experiment and theory. Inorg Chem 49:3836–3850
Dau P, Su J, Liu H, Huang D, Li J, Wang L (2012a) Photoelectron spectroscopy and the electronic structure of the uranyl tetrachloride dianion: UO2Cl4 2−. J Chem Phys 137:064315
Dau P, Su J, Liu H, Liu J, Huang D, Li J, Wang L (2012b) Observation and investigation of the uranyl tetrafluoride dianion (UO2F4 2−) and its solvation complexes with water and acetonitrile. Chem Sci 3:1137–1146
De Bolle F, Moschenborn W (1981) Small system errors of chemical origin in UF isotope measurements during the conversion of the UF6 into UO3 and the reconversion of the UO3 into UF6. Int J Mass Spectrom Ion Phys 38:91–96
Environmental Remediation of Uranium Production Facilities (2005) a joint report by the OECD Nuclear Energy Agency and the International Atomic Energy Agency. OECD Publ, Paris, pp pp. 1–323
European Commission–Directorate General for the Energy-Directorate–Nuclear Safety and Fuel Cycle Radiation Protection, Luxembourg, 2011, pp. 1–13
Esaka F, Lee C, Magara M, Kimura T (2012) Fission track–secondary ion mass spectrometry as a tool for detecting the isotopic signature of individual uranium containing particles. Anal Chim Acta 721:122–128
Engivkann R, de Wolff P (1963) The crystal structure of γ-UO3. Acta Cryst 16:993–996
Frenzel W, Steiner R (1991), Schnellmethgoden zur analyse von plutonium und anderen aktiniden im umweltproben, Fachverband fuer Strahlenschutz e.V., Publikationsreihe, Fortschritte im Strahlenschutz, Verlag TUEV Rheinland, Köln, pp. 1–95.
Frisch M et al (2009) Gaussian 09. Gaussian, Pittsburgh PA, 2009
Geckeis H, Lützenkirchen J, Polly R, Rabung T (2013) Mineral–water interface reactions of actinides. Chem Rev 113(2):1016–1062
Greaves C, Fender B (1972) The structure of α-UO3 by neutron and electron diffraction. Acta Cryst B28:3609–3614
Gorshkov N, Izosimov I, Kazimov A, Kolychev S, Kudryashev N, Mashirov L, Rimskii-Korsakov A, Firsin N (2001) The role of hydroxide ions in reduction of plutonyl ion stimulated by nitrogen laser radiation (337.1 nm). Radiochem 43:360–363
Gresham G, Dinescu A, Benson M, Van Stipdonk M, Groenewold G (2011) Investigation of uranyl nitrate ion pairs complexed with amide ligands using electrospray ionization ion trap mass spectrometry and density functional theory. J Phys Chem A 115:3497–3508
Gross J (2011) Mass spectrometry, a textbook, 2nd edn. Springer, Berlin, pp pp. 1–753
Guerrero J, Gajdosova D, Havel J (2001) Uranium oxide clusters by laser desorption ionization during MALDI-TOF MS analysis of uranium (VI). J Radioanal Nucl Chem 249:139–143
Herebian D, Choi J, Abd El-Aty A, Shim J, Spiteller M (2009) Metabolite analysis in Curcuma domestica using various GC–MS and LC–MS separation and detection techniques. Biomed Chromatogr 23:951–965
Hocking H, Burggraf L, Duan X, Gardella J Jr, Yatzor B, Schuler W (2013) Composition of uranium oxide particles related to TOF-SIMS ion distributions. Surf Interface Anal 45:545–548
Guimbretiere G, Desgranges L, Canizares A, Carlot G, Caraballo R, Jegou C, Duval F, Raimboux N, Ammar M, Simon P (2012) Determination of in-depth damaged profile by Raman line scan in a pre-cut He21 irradiated UO2. Appl Phys Lett 100:251914
Han J, Goncharov V, Kaledin L, Komissarov A, Heaven M (2004) Electronic spectroscopy and ionization potential of UO2 in the gas phase. J Chem Phys 120:5155–5163
Harsha S, Grischkowsky D (2010) Terahertz (far-infrared) characterization of tris(hydroxymethyl)aminomethane using high-resolution waveguide THz-TDS. J Phys Chem A 114:3489–3494
Hildenbrand D (1977) Thermochemistry of gaseous UF5 and UF4. J Chem Phys 66:4788
He H, Qin Z, Shoesmith D (2010) Characterizing the relationship between hyperstoichiometry, defect structure and local corrosion kinetics of uranium dioxide. Electrochim Acta 56:53–60
He H, Wang P, Allred D, Majewski J, Wilkerson M, Rector K (2012) Characterization of chemical speciation in ultrathin uranium oxide layered films. Anal Chem 84:10380–10387
Hillenkamp F, Peter-Katalinic J (2007) (Eds.), MALDI–MS: a practical guide to instrumentation, methods and application, Wiley, New York, 2007, pp. 1–345.
Hu H, Qiu Y, Xiong X, Schwarz W, Li J (2012) On the maximum bond multiplicity of carbon: unusual C ≡ U quadruple bonding in molecular CUO. Chem Sci 3:2786–2796
Isselhardt B, Savina M, Knight K, Pellin M, Hutcheon I, Prussin S (2011) Improving precision in resonance ionization mass spectrometry: influence of laser bandwidth in uranium isotope ratio measurements. Anal Chem 83:2469–2475
Ivanova B, Spiteller M (2012a) A quantitative solid-state Raman spectroscopic method for control of fungicides. Analyst 137:3355–3364
Ivanova B, Spiteller M (2012b) Matrix-assisted laser desorption/ionization mass spectrometric analysis of herbicides in dication-containing organic crystals. Anal Methods 4:4360–436
Ivanova B, Spiteller M (2010a) Noncentrosymmetric crystals with marked nonlinear optical properties. J Phys Chem A 114:5099–5103
Ivanova B, Spiteller M (2010b) On the application of the organic barbiturates as NLO materials. Cryst Growth Des 10:2470–2474
Jung H, Boyanov M, Konishi H, Sun Y, Mishra B, Kemner K, Roden E, Xu H (2012) Redox behavior of uranium at the nanoporous aluminum oxide–Water interface: implications for uranium remediation. Environ Sci Technol 46:7301–7309
Jennings K, Kemp T, Read P (1989) Cluster formation in the fast atom bombardment (FAB) mass spectra of dioxouranium(VI) dinitrate and diacetate. Inorg Chim Acta 157:157–159
Kato R, Rolfe J (1967) Vibration frequencies of NO2 − and NO3 − ions in KBr crystals. J Chem Phys 47:1901–1910
Kalkowski G, Kaindl G, Brewer W, Krone W (1987) Near-edge x-ray-absorption fine structure in uranium compounds. Phys Rev B 35:2667–2677
Kim K, Jung E, Lee K, Cho H, Lee E, Chung D (2012) Evaluation of the behavior of uranium peroxocarbonate complexes in Na–U(VI)–CO3–OH–H2O2 solutions by Raman spectroscopy. J Phys Chem A 116:12024–12031
Kraiema M, Richtera S, Erdmann N, Kuhna H, Hedberg M, Aregbe Y (2012) Characterizing uranium oxide reference particles for isotopic abundances and uranium mass by single particle isotope dilution mass spectrometry. Anal Chim Acta 748:37–44
Koleva B, Kolev T, Spiteller M (2008a) Determination of cephalosporins in solid binary mixtures by polarized IR- and Raman spectroscopy. J Pharmaceut Biomed Anal 48:201–204
Koleva B, Kolev T, Tsalev D, Spiteller M (2008b) Determination of phenacetin and salophen analgetics in solid binary mixtures with caffeine by infrared linear dichroic and Raman spectroscopy. J Pharmaceut Biomed Anal 46:267–273
Koleva B, Kolev T, Simeonov V, Spassov T, Spiteller M (2008c) Linearly polarized IR-spectroscopy of partially oriented solids as a colloid suspension in nematic host: a tool for spectroscopic and structural elucidation of the embedded chemicals. J Incl Phenom Macrocycl Chem 61:319–333
Koleva B, Kolev T, Lamshöft M, Spiteller M (2008d) Synthesis, spectroscopic analysis and structure deduction of gold(III), palladium(II) and platinum(II) complexes with the tripeptide glycyl-l-phenylalanyl-glycine. Trans Met Chem 33:911–919
Kelley C (1999) Iterative methods for optimization, SIAM Frontiers in Applied Mathematics, 18
Knope K, Soderholm L (2013) Solution and solid-state structural chemistry of actinide hydrates and their hydrolysis and condensation products. Chem Rev 113:944–994
Khatib-Shahidi S, Andersson M, Herman J, Gillespie T, Caprioli R (2006) Direct molecular analysis of whole-body animal tissue sections by imaging MALDI mass spectrometry. Anal Chem 78:6448–6456
Kahr M, Abney K, Olivaresc J (2001) Analysis of solid uranium samples using a small mass spectrometer. Spectrochimica Acta Part B 56:1127–1132
Kleinschmidt P, Hildenbrand D (1979) Thermodynamics of the dimerization of gaseous UF5. J Chem Phys 71:196
Lam O, Heinemann F, Meyer K (2011) Activation of elemental S, Se and Te with uranium(III): bridging U–E–U (E ¼ S, Se) and diamond-core complexes U–(E)2–U (E ¼ O, S, Se, Te). Chem Sci 2:1538–1547
Lamshoeft M, Grobe N, Spiteller M (2011) Picomolar concentrations of morphine in human urine determined by dansyl derivatization and liquid chromatography–mass spectrometry. J Chromatogr B 879:933–937
Liang B, Hunt R, Kushto G, Andrews L (2005) Reactions of laser-ablated uranium atoms with H2O in excess argon: a matrix infrared and relativistic DFT investigation of uranium oxyhydrides. Inorg Chem 44:2159–2168
Lipp M, Jenei Z, Klepeis J, Evans W (2007) Raman investigation of the uranium compounds U3O8, UF4, UH3 and UO3 under pressure at room temperature, Lawrence Livermore National Laboratory, LLNL-TR-522251, 1–18.
Madsen K, Nielsen H, Tingleff O (2004) Informatics and mathematical modelling, 2nd edn. DTU, Delhi
Massart D, Vandeginste B, Deming S, Michotte Y, Kaufman L (1988) Chemometrics, vol 2. Elsevier, Amsterdam, pp pp. 1–488
Manna J, Reyzer M, Latham J, Weaver C, Marnett L, Caprioli R (2011) High-throughput quantification of bioactive lipids by MALDI mass spectrometry: application to prostaglandins. Anal Chem 83:6683–6688
Monge M, Harris G, Dwivedi P, Fernandez F (2013) Mass spectrometry: recent advances in direct open air surface sampling/ionization. Chem Rev 113:2269–2308
Mayer K, Wallenius M, Varga Z (2013) Nuclear forensic science: correlating measurable material parameters to the history of nuclear material. Chem Rev 113:884–900
Mennucci B, Tomasi J, Cammi R, Cheeseman J, Frisch M, Devlin F, Gabriel S, Stephens P (2002) Polarizable continuum model (PCM) calculations of solvent effects on optical rotations of chiral molecules. J Phys Chem A 106:6102–6113
McGlynn S, Smith J, Neely W (1961) Electronic structure, spectra, and magnetic properties of oxycations. III. Ligation effects on the infrared spectrum of the uranyl ion. J Chem Phys 35:105–116
Norris J, Caprioli R (2013) Analysis of tissue specimens by matrix-assisted laser desorption/ionization imaging mass spectrometry in biological and clinical research. Chem Rev 113:2309–2342
Nebbioso A, Piccolo A, Spiteller M (2010) Limitations of electrospray ionization in the analysis of a heterogeneous mixture of naturally occurring hydrophilic and hydrophobic compounds. Rapid Commun Mass Spectrom 24:3163–3170
Nguyen-Trung C, Begun G, Palmer D (1992) Aqueous uranium complexes. 2. Raman spectroscopic study of the complex formation of the dioxouranium(VI) ion with a variety of inorganic and organic ligands. Inorg Chem 31:5280–5287
Oppenheim K, Korter T, Melinger J, Grischkowsky D (2010) Solid-state density functional theory investigation of the terahertz spectra of the structural isomers 1,2-dicyanobenzene and 1,3-dicyanobenzene. J Phys Chem A 114:12513–12521
Park J, Choi I, Park S, Lee M, Song K (2011) A correction method for the peak tailing backgrounds for accurate isotope ratio measurements of uranium in ultra trace levels using thermal ionization mass spectrometry. Bull Korean Chem Soc 32:4327–4331
Pan Q, Schreckenbach G (2010) Binuclear hexa- and pentavalent uranium complexes with a polypyrrolic ligand: a density functional study of water- and hydronium-induced reactions. Inorg Chem 49:6509–6517
Plasil J, Buixaderas E, Cejka J, Sejkora J, Jehlicka J, Novak M (2010) Raman spectroscopic study of the uranyl sulphate mineral zippeite: low wavenumber and U–O stretching regions. Anal Bioanal Chem 397:2703–2715
Petiau J, Calas G, Petitmaire D, Bianconi A, Benfatto M, Marcelli A (1986) Delocalized versus localized unoccupied 5f states and the uranium site structure in uranium oxides and glasses probed by x-ray-absorption near-edge structure. Phys Rev B 34:7350–7361
Perez-Bendito D, Rubio S (1999) Environmental analytical chemistry, vol XXXII, Wilson and Wilson's comprehensive analytical chemistry. Elsevier, Amsterdam, pp pp.1–842
Pasilis S, Blumenfeld A (2011) Effect of nitrate, perchlorate, and water on uranyl(VI) speciation in a room-temperature ionic liquid: a spectroscopic investigation. Inorg Chem 50:8302–8307
Qiu J, Burns P (2013) Clusters of actinides with oxide, peroxide, or hydroxide bridges. Chem Rev 2013(113):1097–1120
Roof R, Cromer J, Larson A (1964) The crystal structure of uranyl fihydroxide, UO2(OH)2 *. Acta Cryst 17:701–705
Richter S, Kuehn H, Truyens J, Kraiem M, Aregbea Y (2013) Uranium hexafluoride (UF6) gas source mass spectrometry for certification of reference materials and nuclear safeguard measurements at IRMM. J Anal At Spectrom 28:536–548
Shuvalov R, Burns P (2003) A monoclinic polymorph of uranyl dinitrate trihydrate, [UO2(NO3)2(H2O)2]·H2O. Acta Cryst C59:i71–i73
Siegel S, Hoekstra H, Gebert E (1972) The structure of γ-uranyl dihydroxide, UO2(OH)2 *. Acta Cryst B28:3469–3473
Stanley F (2012) A beginner's guide to uranium chronometry in nuclear forensics and safeguards. J Anal At Spectrom 27:1821–1830
Saprygina A, Elistratova O, Kalashnikova V, Kulika I, Rodicheva I (2011) Mass spectrometry determination of the isotopic composition of uranium hexafluoride. J Anal Chem 66:1385–1391
Spiteller M (1985) Extraction of soil organic matter by supercritical fluids. Org Geochem 8:111–113
Spiteller M (1987) Isolation and characterisation of dissolved organic carbon from natural and lysimeter waters by ultrafiltration. Sci Tot Environm 62:47–54
Stoliker D, Kent D, Zachara J (2011) Quantifying differences in the impact of variable chemistry on equilibrium uranium(VI) adsorption properties of aquifer sediments. Environ Sci Technol 45:8733–8740
Schlüsener M, Bester K, Spiteller M (2003) Determination of antibiotics such as macrolides, ionophores and tiamulin in liquid manure by HPLC–MS/MS. Anal Bioanal Chem 375:942–947
Toth L, Begun G (1981) Raman spectra of uranyl ion and its hydrolysis products in aqueous HNO3. J Phys Chem 85:547–549
Stuke M, Wittig C (1981) Multiply charged atomic and molecular ions from laser multiphoton ionization of UF6. Chem Phys Lett 81:168–169
Stuke M, Reisler H, Wittig C (1981) Monitoring UF6 photodissociation via laser multiphoton ionization. Appl Phys Lett 39:201
Tretyakova N, Villalta P, Kotapati S (2013) Mass spectrometry of structurally modified DNA. Chem Rev 113:2395–2436
Vidjayacoumar B, Ilango S, Ray M, Chu T, Kolpin K, Andreychuk N, Cruz C, Emslie D, Jenkins H, Britten J (2012) Rigid NON- and NSN-ligand complexes of tetravalent and trivalent uranium: comparison of U–OAr2 and U–SAr2 bonding. Dalton Trans 41:8175–8189
Vallet V, Wahlgren U, Grenthe I (2012) Probing the nature of chemical bonding in uranyl(VI) complexes with quantum chemical methods. J Phys Chem A 116:12373–12380
Van Stipdonk M, Chien W, Bulleigh K, Wu Q, Groenewold D (2006) Gas-phase uranyl–nitrile complex ions. J Phys Chem A 110:959–970
Walton S, Mitchell D (2013) A novel rapid detection approach for the analysis of radionuclides in environmental samples using graphite MALDI mass spectrometry, J Radioanal Nucl Chem DOI 10.1007/s10967-012-2176-1
Wang D, van Gunsteren W, Chai Z (2012) Recent advances in computational actinoid chemistry. Chem Soc Rev 41:5836–5865
Weller M, Light M, Gelbrich T (2000) Structure of uranium(VI) oxide dihydrate, UO3·2H2O; synthetic meta-schoepite (UO2)4O(OH)6·5H2O. Acta Cryst B56:577–583
Yang J, Caprioli R (2011) Matrix sublimation/recrystallization for imaging proteins by mass spectrometry at high spatial resolution. Anal Chem 83:5728–5734
Zalazar M, Rayon V, Largo A (2012) On the molecular structure of uranium dicarbide: T-shape versus linear isomers. J Phys Chem A 116:2972–2977
Zhao Y, Truhlar D (2008a) Density functionals with broad applicability in chemistry. Accts Chem Res 41:157–167
Zhao Y, Truhlar D (2008b) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241
Acknowledgments
The authors thank the Deutscher Akademischer Austausch Dienst, the Deutsche Forschungsgemeinschaft, the central instrumental laboratories for structural analysis at Dortmund University (Federal State Nordrhein–Westfalen, Germany), and the analytical and computational laboratory cluster at the Institute of Environmental Research (INFU) at the same University.
Conflict of interest
Michael Spiteller has received a research grant (Deutsche Forschungsgemeinschaft 255/22-1). Bojidarka Ivanova has received research grant (Deutsche Forschungsgemeinschaft 255/22-1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 142 kb)
Rights and permissions
About this article
Cite this article
Ivanova, B., Spiteller, M. Uranyl–water-containing complexes: solid-state UV-MALDI mass spectrometric and IR spectroscopic approach for selective quantitation. Environ Sci Pollut Res 21, 1548–1563 (2014). https://doi.org/10.1007/s11356-013-1892-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1892-6