Abstract
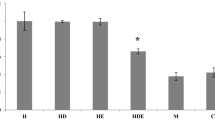
The aim of this study was to evaluate lung histology and the levels of HIF-1α protein in response to the reduction in training volume for 14 and 21 days with Nigella sativa (NS) supplementation. 60 three-week-old male Wistar rats (68 ± 9 g) were divided into 6 groups after 6 weeks of incremental interval training (IIT); IIT control and IIT groups, 2 groups of 14-day taper (with and without NS) and, similarly, 2 groups of 21-day taper. After 6 weeks, NS seed ethanol extract was fed daily by gavages to NS groups at 500 mg/kg. Finally, animals were euthanized and their lungs were removed, weighed and fixed in 10 % formalin. By routine and standard paraffin embedding, lung samples were sectioned in 5 μm and stained with H&E and studied histologically. The level of HIF-1α was measured by ELISA kit. The results showed that the administration of NS and 21-day taper in the IIT exposed rats caused significant difference in lung weight and lung HIF-1α (p = 0.001, p = 0.001). But the interaction between groups and taper periods was not confirmed (p = 0.530). Lung histology in 21-day taper with NS was better than the other groups. The results showed that after IIT, 21-day reduction in training volume and the use of new strategies such as NS supplementation in this period can reduce HIF-1α higher amounts in the lungs, which may probably be due to the anti-inflammatory effects of the NS supplement. These agents can improve respiratory capacity for competition or severe activity after taper period.



Similar content being viewed by others
Abbreviations
- NS:
-
Nigella sativa
- IIT:
-
Incremental interval training
References
Le Meur Y, Hausswirth C, Mujika I (2012) Tapering for competition: a review. Sci Sport 27(2):77–87
Mujika I (2010) Intense training: the key to optimal performance before and during the taper. Scand J Med Sci Sports 20(s2):24–31
Rietjens G, Keizer H, Kuipers H et al (2001) A reduction in training volume and intensity for 21 days does not impair performance in cyclists. Br J Sports Med 35(6):431–434
Mujika I, Chaouachi A, Chamari K (2010) Precompetition taper and nutritional strategies: special reference to training during Ramadan intermittent fast. Br J Sports Med 44(7):495–501
Farhangi N, Zehsaz F (2010) The effect of the tapering on the concentration of some plasma cytokines and physical performance in endurance male runners. JBUMS 12:51–58
Bosquet L, Montpetit J, Arvisais D et al (2007) Effects of tapering on performance: a meta-analysis. Med Sci Sports Exerc 39(8):1358–1365
Papacosta E, Gleeson M (2013) Effects of intensified training and taper on immune function. Rev Bras Educ Fís Esporte 27(1):159–176
Dehkordi KJ, Ebrahim K, Gaeini A et al (2014) The effect of two types of tapering on cortisol, testosterone and testosterone/cortisol ratio in male soccer players. Int J Basic Sci Appl Res 3(2):79–84
Farhangimaleki N, Zehsaz F, Tiidus PM (2009) The effect of tapering period on plasma pro-inflammatory cytokine levels and performance in elite male cyclists. J Sports Sci Med 8(4):600–606
Mujika I, Padilla S (2003) Scientific bases for precompetition tapering strategies. Med Sci Sports Exerc 35(7):1182–1187
Thomas L, Busso T (2005) A theoretical study of taper characteristics to optimize performance. Med Sci Sports Exerc 37(9):1615–1621
Pedersen BK, Steensberg A (2002) Exercise and hypoxia: effects on leukocytes and interleukin-6-shared mechanisms? Med Sci Sports Exerc 34(12):2004–2012
Ramakrishnan S, Anand V, Roy S (2014) Vascular endothelial growth factor signaling in hypoxia and inflammation. J Neuroimmune Pharmacol 9(2):142–160
Scholz CC, Taylor CT (2013) Targeting the HIF pathway in inflammation and immunity. Curr Opin Pharmacol 13(4):646–653
Dayan F, Mazure NM, Brahimi-Horn MC et al (2008) A dialogue between the hypoxia-inducible factor and the tumor microenvironment. Cancer Microenviron 1(1):53–68
Koh MY, Powis G (2012) Passing the baton: the HIF switch. Trends Biochem Sci 37(9):364–372
Olmeda B, Umstead TM, Silveyra P et al (2014) Effect of hypoxia on lung gene expression and proteomic profile: insights into the pulmonary surfactant response. J Proteomics 101:179–191
Semenza GL (2012) Hypoxia-inducible factors in physiology and medicine. Cell 148(3):399–408
Brusselmans K, Compernolle V, Tjwa M et al (2003) Heterozygous deficiency of hypoxia-inducible factor-2α protects mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia. J Clin Invest 111(10):1519–1527
Krick S, Eul BG, Hanze J et al (2005) Role of hypoxia-inducible factor-1α in hypoxia-induced apoptosis of primary alveolar epithelial type II cells. Am J Respir Cell Mol Biol 32(5):395–403
Bove PF, Hristova M, Wesley UV et al (2008) Inflammatory levels of nitric oxide inhibit airway epithelial cell migration by inhibition of the kinase ERK1/2 and activation of hypoxia-inducible factor-1α. J Biol Chem 283(26):17919–17928
Asfour W, Almadi S, Haffar L (2013) Thymoquinone suppresses cellular proliferation, inhibits VEGF production and obstructs tumor progression and invasion in the rat model of DMH-induced colon carcinogenesis. Pharmacol Pharm 4(1):7–17
Gilani A, Jabeen Q, Khan M (2004) A review of medicinal uses and pharmacological activities of Nigella sativa. Pak J Biol Sci 7:441–451
Boskabady MH, Vahedi N, Amery S et al (2011) The effect of Nigella sativa alone, and in combination with dexamethasone, on tracheal muscle responsiveness and lung inflammation in sulfur mustard exposed guinea pigs. J Ethnopharmacol 137(2):1028–1034
Khan MA (1999) Chemical composition and medicinal properties of Nigella sativa Linn. Inflammopharmacology 7(1):15–35
Modaresi M (2012) The effect of black seed (Nigella sativa) hydro-alcoholic extract on breeding factors in female mice. J Shahrekord Univ Med Sci 13(6):63–70
Gali-Muhtasib H, El-Najjar N, Schneider-Stock R (2006) The medicinal potential of black seed (Nigella sativa) and its components. Adv Phytomedicine 2:133–153
Kalus U, Pruss A, Bystron J et al (2003) Effect of Nigella sativa (black seed) on subjective feeling in patients with allergic diseases. Phytother res 17(10):1209–1214
Ahmad A, Husain A, Mujeeb M et al (2013) A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pac J Trop Biomed 3(5):337–352
Ogura Y, Naito H, Kurosaka M et al (2006) Sprint-interval training induces heat shock protein 72 in rat skeletal muscles. J Sports Sci Med 5(2):194–201
Al-Ghamdi MS (2003) Protective effect of Nigella sativa seeds against carbon tetrachloride-induced liver damage. Am J Chin Med 31(05):721–728
Medeiros RMV, Arrais RF, Azevedo JCVd et al (2014) Discriminant analysis of pubertal maturation in young males based on anthropometric characteristics. Rev Bras Cineantropom Desempenho Hum 16(1):96–105
Rogol AD, Roemmich JN, Clark PA (2002) Growth at puberty. J Adolesc Health 31(6):192–200
Izquierdo-gabarren M, Izquierdo M (2010) Physiological effects of tapering and detraining in world-class kayakers. Med Sci Sports Exerc 42(6):1209–1214
Sultan MT, Butt MS, Ahmad RS et al (2011) Supplementation of powdered black cumin (Nigella sativa) seeds reduces the risk of hypercholesterolemia. Funct Food Health Dis 12:516–524
Tasawar Z, Siraj Z, Ahmad N et al (2011) The effects of Nigella sativa (Kalonji) on lipid profile in patients with stable coronary artery disease in Multan Pakistan. Pak J Nutr 10(2):162–167
Parhizkar S, Latiffah A, Sabariah A et al (2011) Preventive effect of Nigella sativa on metabolic syndrome in menopause induced rats. J Med Plants Res 5(8):1478–1484
Meddah B, Ducroc R, El Abbes Faouzi M et al (2009) Nigella sativa inhibits intestinal glucose absorption and improves glucose tolerance in rats. J Ethnopharmacol 121(3):419–424
Zaoui A, Cherrah Y, Alaoui K et al (2002) Effects of Nigella sativa fixed oil on blood homeostasis in rat. J Ethnopharmacol 79(1):23–26
Buriro MA, Tayyab M (2007) Effect of Nigella sativa on lipid profile in albino rats. Gomal J Med Sci 5(1):28–31
Benhaddou-Andaloussi A, Martineau L, Vuong T et al (2011) The in vivo antidiabetic activity of Nigella sativa is mediated through activation of the AMPK pathway and increased muscle Glut4 content. Evid Based Complement Alternat Med 2011:1–9
Mason SD, Howlett RA, Kim MJ et al (2004) Loss of skeletal muscle HIF-1α results in altered exercise endurance. PLoS Biol 2(10):e288:1540–1548
Mounier R, Pialoux V, Roels B et al (2009) Effect of intermittent hypoxic training on HIF gene expression in human skeletal muscle and leukocytes. Eur J Appl Physiol 105(4):515–524
Yu AY, Frid MG, Shimoda LA et al (1998) Temporal, spatial, and oxygen-regulated expression of hypoxia-inducible factor-1 in the lung. Am J Physiol Lung Cell Mol Physiol 275(4):818–826
Gharaee-Kermani M, Gyetko MR, Hu B et al (2007) New insights into the pathogenesis and treatment of idiopathic pulmonary fibrosis: a potential role for stem cells in the lung parenchyma and implications for therapy. Pharm Res 24(5):819–841
He X, Shi X, Yuan H et al (2012) Propofol attenuates hypoxia-induced apoptosis in alveolar epithelial type II cells through down-regulating hypoxia-inducible factor-1α. Injury 43(3):279–283
Ke Q, Costa M (2006) Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol 70(5):1469–1480
Uhal BD, Gidea C, Bargout R et al (1998) Captopril inhibits apoptosis in human lung epithelial cells: a potential antifibrotic mechanism. Am J Physiol Lung Cell Mol Physiol 275(5):1013–1017
Han F, Luo Y, Li Y et al (2012) Seawater induces apoptosis in alveolar epithelial cells via the Fas/FasL-mediated pathway. Respir Physiol Neurobiol 182(2):71–80
Yeh C-H, Cho W, So EC et al (2011) Propofol inhibits lipopolysaccharide-induced lung epithelial cell injury by reducing hypoxia-inducible factor-1α expression. Br J Anaesth 106(4):590–599
El-Khouly D, El-Bakly WM, Awad A et al (2012) Thymoquinone blocks lung injury and fibrosis by attenuating bleomycin-induced oxidative stress and activation of nuclear factor Kappa-B in rats. Toxicology 302(2–3):106–113
Asim M, Shahzad M, Yang X et al (2010) Suppressive effects of black seed oil on ovalbumin induced acute lung remodeling in E3 rats. Swiss Med Wkly 140(w13128):1–7
Acknowledgments
We are grateful to Mehdi Hedayati and Gholamreza Hamidian for their analytical help. We also acknowledge the Department of Exercise Physiology at the University of Mazandaran for their contributions.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was conducted according to the Declaration of Helsinki guidelines and approved by the Ethical Committee of the University of Mazandaran.
Informed consent
Informed consent was obtained from all subjects for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arabzadeh, E., Mirdar, S. & Fathi, Z. Measurement of levels of lung HIF-1α protein in response to tapering for 14- and 21-day with nigella sativa supplementation in maturing rat, with histological study. Sport Sci Health 11, 195–202 (2015). https://doi.org/10.1007/s11332-015-0223-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-015-0223-3