Abstract
Background
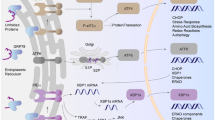
Obstructive sleep apnea syndrome, which is a risk factor for resistant hypertension, is characterized by chronic intermittent hypoxia (CIH) and is associated with many cardiovascular diseases. CIH elicits systemic oxidative stress and sympathetic hyperactivity, which lead to hypertension. Rho kinases (ROCKs) are considered to be major effectors of the small GTPase RhoA and have been extensively studied in the cardiovascular field. Upregulation of the RhoA/ROCK signaling cascade is observed in various cardiovascular disorders, such as atherosclerosis, pulmonary hypertension, and stroke. However, the exact molecular function of RhoA/ROCK in CIH remains unclear and requires further study.
Objective
This study aimed to investigate the role of the NADPH oxidase 4 (Nox4)-induced ROS/RhoA/ROCK pathway in CIH-induced hypertension in rats.
Methods
Male Sprague–Dawley rats were exposed to CIH for 21 days (intermittent hypoxia of 21% O2 for 60 s and 5% O2 for 30 s, cyclically repeated for 8 h/day). We randomly assigned 56 male rats to groups of normoxia (RA) or vertically implemented CIH together with vehicle (CIH-V), GKT137831 (CIH-G), N-acetyl cysteine (NAC) (CIH-N), or Y27632 (CIH-Y). The rats in the RA group were continuously exposed to room air, whereas the rats in the other groups were exposed to CIH. Systolic blood pressure (BP) was monitored at the beginning of each week. After the experiment, renal sympathetic nerve activity (RSNA) was recorded, and serum and renal tissues were subjected to molecular biological and biochemical analyses.
Results
Compared with the BP of RA rats, the BP of CIH-V rats started to increase 2 weeks after the beginning of the experiment, subsequently stabilizing at a high level at the end of the third week. CIH increased both RSNA and oxidative stress. This response was attenuated by treatment of the rats with GKT137831 or NAC. Inhibiting Nox4 activity or ROS production reduced RhoA/ROCK expression. Treatment with Y27632 reduced both BP and RSNA in rats exposed to CIH.
Conclusion
Hypertension can be induced by CIH in SD rats. The CIH-induced elevation of BP is at least partially mediated via the Nox4-induced ROS/RhoA/ROCK pathway.







Similar content being viewed by others
References
Mooe T, Franklin KA, Wiklund U, Rabben T, Holmstrom K (2000) Sleep-disordered breathing and myocardial ischemia in patients with coronary artery disease. Chest 117:1597–1602
Calhoun DA (2010) Obstructive sleep apnea and hypertension. Curr Hypertens Rep 12:189–195
Prabhakar NR, Kumar GK (2010) Mechanisms of sympathetic activation and blood pressure elevation by intermittent hypoxia. Respir Physiol Neurobiol 174:156–161
Xing T, Pilowsky PM, Fong AY (2014) Mechanism of sympathetic activation and blood pressure elevation in humans and animals following acute intermittent hypoxia. Prog Brain Res 209:131–146
Peng YJ, Yuan G, Khan S, Nanduri J, Makarenko VV, Reddy VD et al (2014) Regulation of hypoxia-inducible factor-alpha isoforms and redox state by carotid body neural activity in rats. J Physiol 592:3841–3858
Prabhakar NR, Peng YJ, Kumar GK, Nanduri J (2015) Peripheral chemoreception and arterial pressure responses to intermittent hypoxia. Compr Physiol 5:561–577
Eckardt KU, Bernhardt WM, Weidemann A, Warnecke C, Rosenberger C, Wiesener MS, et al. (2005) Role of hypoxia in the pathogenesis of renal disease. Kidney Int SupplS46-S51
Loirand G, Pacaud P (2010) The role of Rho protein signaling in hypertension. Nat Rev Cardiol 7:637–647
Nguyen DCA, Touyz RM (2011) Cell signaling of angiotensin II on vascular tone: novel mechanisms. Curr Hypertens Rep 13:122–128
Nunes KP, Rigsby CS, Webb RC (2010) RhoA/Rho-kinase and vascular diseases: what is the link? Cell Mol Life Sci 67:3823–3836
Mukai Y, Shimokawa H, Matoba T, Kandabashi T, Satoh S, Hiroki J et al (2001) Involvement of Rho-kinase in hypertensive vascular disease: a novel therapeutic target in hypertension. FASEB J 15:1062–1064
Moriki N, Ito M, Seko T, Kureishi Y, Okamoto R, Nakakuki T et al (2004) RhoA activation in vascular smooth muscle cells from stroke-prone spontaneously hypertensive rats. Hypertens Res 27:263–270
Masumoto A, Hirooka Y, Shimokawa H, Hironaga K, Setoguchi S, Takeshita A (2001) Possible involvement of Rho-kinase in the pathogenesis of hypertension in humans. Hypertension 38:1307–1310
Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T et al (1997) Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389:990–994
Dikalov SI, Ungvari Z (2013) Role of mitochondrial oxidative stress in hypertension. Am J Physiol Heart Circ Physiol 305:H1417–H1427
Dumitrascu R, Heitmann J, Seeger W, Weissmann N, Schulz R (2013) Obstructive sleep apnea, oxidative stress and cardiovascular disease: lessons from animal studies. Oxidative Med Cell Longev 2013:234631
Harrison DG, Gongora MC (2009) Oxidative stress and hypertension. Med Clin North Am 93:621–635
O’Halloran KD (2015) Chronic intermittent hypoxia creates the perfect storm with calamitous consequences for respiratory control. Respir Physiol Neurobiol
Rubattu S, Pagliaro B, Pierelli G, Santolamazza C, Castro SD, Mennuni S et al (2015) Pathogenesis of target organ damage in hypertension: role of mitochondrial oxidative stress. Int J Mol Sci 16:823–839
Denniss SG, Jeffery AJ, Rush JW (2010) RhoA-Rho kinase signaling mediates endothelium- and endoperoxide-dependent contractile activities characteristic of hypertensive vascular dysfunction. Am J Physiol Heart Circ Physiol 298:H1391–H1405
Schluter T, Steinbach AC, Steffen A, Rettig R, Grisk O (2008) Apocynin-induced vasodilation involves Rho kinase inhibition but not NADPH oxidase inhibition. Cardiovasc Res 80:271–279
Sun Q, Yue P, Ying Z, Cardounel AJ, Brook RD, Devlin R et al (2008) Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK. Arterioscler Thromb Vasc Biol 28:1760–1766
Jin L, Ying Z, Webb RC (2004) Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol 287:H1495–H1500
Feng SZ, Tian JL, Zhang Q, Wang H, Sun N, Zhang Y et al (2011) An experimental research on chronic intermittent hypoxia leading to liver injury. Sleep Breath 15:493–502
Idiaquez J, Santos I, Santin J, Del RR, Iturriaga R (2014) Neurobehavioral and autonomic alterations in adults with obstructive sleep apnea. Sleep Med 15:1319–1323
Iturriaga R, Andrade DC, Del RR (2014) Enhanced carotid body chemosensory activity and the cardiovascular alterations induced by intermittent hypoxia. Front Physiol 5:468
Marcus JA, Pothineni A, Marcus CZ, Bisognano JD (2014) The role of obesity and obstructive sleep apnea in the pathogenesis and treatment of resistant hypertension. Curr Hypertens Rep 16:411
Floras JS (2015) Hypertension and sleep apnea. Can J Cardiol 31:889–897
Levy P, Tamisier R, Arnaud C, Monneret D, Baguet JP, Stanke-Labesque F et al (2012) Sleep deprivation, sleep apnea and cardiovascular diseases. Front Biosci (Elite Ed) 4:2007–2021
Del RR, Moya EA, Parga MJ, Madrid C, Iturriaga R (2012) Carotid body inflammation and cardiorespiratory alterations in intermittent hypoxia. Eur Respir J 39:1492–1500
Peng YJ, Raghuraman G, Khan SA, Kumar GK (1985) Prabhakar NR (2011) angiotensin II evokes sensory long-term facilitation of the carotid body via NADPH oxidase. J Appl Physiol 111:964–970
Parati G, Lombardi C, Hedner J, Bonsignore MR, Grote L, Tkacova R et al (2013) Recommendations for the management of patients with obstructive sleep apnoea and hypertension. Eur Respir J 41:523–538
Silva AQ, Schreihofer AM (2011) Altered sympathetic reflexes and vascular reactivity in rats after exposure to chronic intermittent hypoxia. J Physiol 589:1463–1476
Denton KM, Shweta A, Anderson WP (2002) Preglomerular and postglomerular resistance responses to different levels of sympathetic activation by hypoxia. J Am Soc Nephrol 13:27–34
Linz D, Mahfoud F, Linz B, Hohl M, Schirmer SH, Wirth KJ et al (2014) Effect of obstructive respiratory events on blood pressure and renal perfusion in a pig model for sleep apnea. Am J Hypertens 27:1293–1300
Linz D, Mahfoud F, Schotten U, Ukena C, Neuberger HR, Wirth K et al (2012) Renal sympathetic denervation suppresses postapneic blood pressure rises and atrial fibrillation in a model for sleep apnea. Hypertension 60:172–178
Bao G, Metreveli N, Li R, Taylor A (1985) Fletcher EC (1997) blood pressure response to chronic episodic hypoxia: role of the sympathetic nervous system. J Appl Physiol 83:95–101
Fletcher EC, Bao G, Li R (1999) Renin activity and blood pressure in response to chronic episodic hypoxia. Hypertension 34:309–314
Huang J, Lusina S, Xie T, Ji E, Xiang S, Liu Y et al (2009) Sympathetic response to chemostimulation in conscious rats exposed to chronic intermittent hypoxia. Respir Physiol Neurobiol 166:102–106
Somlyo AP, Somlyo AV (2004) Signal transduction through the RhoA/Rho-kinase pathway in smooth muscle. J Muscle Res Cell Motil 25:613–615
Berridge MJ (2008) Smooth muscle cell calcium activation mechanisms. J Physiol 586:5047–5061
Urena J, Lopez-Barneo J (2012) Metabotropic regulation of RhoA/Rho-associated kinase by L-type Ca2+ channels. Trends Cardiovasc Med 22:155–160
Firth AL, Choi IW, Park WS (2012) Animal models of pulmonary hypertension: Rho kinase inhibition. Prog Biophys Mol Biol 109:67–75
Lee TM, Chung TH, Lin SZ, Chang NC (2014) Endothelin receptor blockade ameliorates renal injury by inhibition of RhoA/Rho-kinase signalling in deoxycorticosterone acetate-salt hypertensive rats. J Hypertens 32:795–805
de Frutos S, Caldwell E, Nitta CH, Kanagy NL, Wang J, Wang W et al (2010) NFATc3 contributes to intermittent hypoxia-induced arterial remodeling in mice. Am J Physiol Heart Circ Physiol 299:H356–H363
Allahdadi KJ, Walker BR, Kanagy NL (2007) ROK contribution to endothelin-mediated contraction in aorta and mesenteric arteries following intermittent hypoxia/hypercapnia in rats. Am J Physiol Heart Circ Physiol 293:H2911–H2918
Ito K, Hirooka Y, Kimura Y, Shimokawa H, Takeshita A (2005) Effects of hydroxyfasudil administered to the nucleus tractus solitarii on blood pressure and heart rate in spontaneously hypertensive rats. Clin Exp Hypertens 27:269–277
Ito K, Hirooka Y, Kishi T, Kimura Y, Kaibuchi K, Shimokawa H et al (2004) Rho/Rho-kinase pathway in the brainstem contributes to hypertension caused by chronic nitric oxide synthase inhibition. Hypertension 43:156–162
Ito K, Hirooka Y, Sakai K, Kishi T, Kaibuchi K, Shimokawa H et al (2003) Rho/Rho-kinase pathway in brain stem contributes to blood pressure regulation via sympathetic nervous system: possible involvement in neural mechanisms of hypertension. Circ Res 92:1337–1343
Cui Q, Zhang Y, Chen H, Li J (2013) Rho kinase: a new target for treatment of cerebral ischemia/reperfusion injury. Neural Regen Res 8:1180–1189
Noma K, Kihara Y, Higashi Y (2012) Striking crosstalk of ROCK signaling with endothelial function. J Cardiol 60:1–6
Sari AN, Kacan M, Unsal D, Sahan FS, Kemal BC, Vezir O et al (2014) Contribution of RhoA/Rho-kinase/MEK1/ERK1/2/iNOS pathway to ischemia/reperfusion-induced oxidative/nitrosative stress and inflammation leading to distant and target organ injury in rats. Eur J Pharmacol 723:234–245
Satoh K, Fukumoto Y, Shimokawa H (2011) Rho-kinase: important new therapeutic target in cardiovascular diseases. Am J Physiol Heart Circ Physiol 301:H287–H296
Shi J, Wei L (2013) Rho kinases in cardiovascular physiology and pathophysiology: the effect of fasudil. J Cardiovasc Pharmacol 62:341–354
Lassegue B, San MA, Griendling KK (2012) Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res 110:1364–1390
Selemidis S, Sobey CG, Wingler K, Schmidt HH, Drummond GR (2008) NADPH oxidases in the vasculature: molecular features, roles in disease and pharmacological inhibition. Pharmacol Ther 120:254–291
Gorin Y, Block K (2013) Nox4 and diabetic nephropathy: with a friend like this, who needs enemies? Free Radic Biol Med 61:130–142
Gorin Y, Wauquier F (2015) Upstream regulators and downstream effectors of NADPH oxidases as novel therapeutic targets for diabetic kidney disease. Mol Cells 38:285–296
Lassegue B, Griendling KK (2010) NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol 30:653–661
Nistala R, Whaley-Connell A, Sowers JR (2008) Redox control of renal function and hypertension. Antioxid Redox Signal 10:2047–2089
Eid AA, Lee DY, Roman LJ, Khazim K, Gorin Y (2013) Sestrin 2 and AMPK connect hyperglycemia to Nox4-dependent endothelial nitric oxide synthase uncoupling and matrix protein expression. Mol Cell Biol 33:3439–3460
Cuevas S, Zhang Y, Yang Y, Escano C, Asico L, Jones JE et al (2012) Role of renal DJ-1 in the pathogenesis of hypertension associated with increased reactive oxygen species production. Hypertension 59:446–452
Jha JC, Gray SP, Barit D, Okabe J, El-Osta A, Namikoshi T et al (2014) Genetic targeting or pharmacologic inhibition of NADPH oxidase nox4 provides renoprotection in long-term diabetic nephropathy. J Am Soc Nephrol 25:1237–1254
Chi AY, Waypa GB, Mungai PT, Schumacker PT (2010) Prolonged hypoxia increases ROS signaling and RhoA activation in pulmonary artery smooth muscle and endothelial cells. Antioxid Redox Signal 12:603–610
Knock GA, Snetkov VA, Shaifta Y, Connolly M, Drndarski S, Noah A et al (2009) Superoxide constricts rat pulmonary arteries via rho-kinase-mediated Ca(2+) sensitization. Free Radic Biol Med 46:633–642
Jackson EK, Gillespie DG, Zhu C, Ren J, Zacharia LC, Mi Z (2008) Alpha2-adrenoceptors enhance angiotensin II-induced renal vasoconstriction: role for NADPH oxidase and RhoA. Hypertension 51:719–726
Gorin Y, Cavaglieri RC, Khazim K, Lee DY, Bruno F, Thakur S et al (2015) Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. Am J Physiol Renal Physiol 308:F1276–F1287
Acknowledgements
We thank Dr. Jing Feng and Prof. Baoyuan Chen, Respiratory Department, Tianjin Medical University General Hospital, China, for their support with the intermittent hypoxia chamber and the gas control delivery system used in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The National Natural Science Foundation of China provided financial support in the form of national natural science funding (No.81070065, 81370181). The sponsor had no role in the design or conduct of this research.
Conflict of interest
The authors declare that they have no conflict of interest.
Animal experiments
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Author contributions
WL and JK: Directly participated in the execution of the study and molecular biology experiment
KH: Study planning, analysis of the study and writing of the manuscript
ST, XZ, LX, YL, and SY: CIH exposure of rats and gas control delivery
Rights and permissions
About this article
Cite this article
Lu, W., Kang, J., Hu, K. et al. The role of the Nox4-derived ROS-mediated RhoA/Rho kinase pathway in rat hypertension induced by chronic intermittent hypoxia. Sleep Breath 21, 667–677 (2017). https://doi.org/10.1007/s11325-016-1449-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-016-1449-2