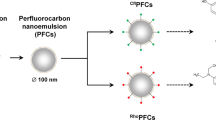
Abstract
Purpose
We aim to develop perfluorocarbon-based nanoemulsions with improved sensitivity for detection of inflammatory macrophages in situ using F-19 MRI. Towards this goal, we evaluate the feasibility of nanoemulsion formulation incorporating a metal chelate in the fluorous phase which shortens the F-19 longitudinal relaxation rate and image acquisition time.
Procedures
Perfluorinated linear polymers were conjugated to metal-binding tris-diketonate, blended with unconjugated polymers, and emulsified in water. Phospholipid-based surfactant was used to stabilize nanoemulsion and provide biocompatibility. Nanoemulsions were metalated with the addition of ferric salt to the buffer. Physical stability of surfactant and nanoemulsion was evaluated by mass spectrometry and dynamic light scattering measurements. Nanoemulsions were injected intravenously into a murine granuloma inflammation model, and in vivo19F/1H MRI at 11.7 T was performed.
Results
We demonstrated stability and biocompatibility of lipid-based paramagnetic nanoemulsions. We investigated potential oxidation of lipid in the presence of metal chelate. As a proof of concept, we performed non-invasive monitoring of macrophage burden in a murine inflammation model following intravenous injection of nanoemulsion using in vivo F-19 MRI.
Conclusion
Lipid-based nanoemulsion probes of perfluorocarbon synthesized with iron-binding fluorinated β-diketones can be formulated for intravenous delivery and inflammation detection in vivo.






Similar content being viewed by others
Change history
30 September 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11307-021-01643-8
References
Ahrens ET, Zhong J (2013) In vivo MRI cell tracking using perfluorocarbon probes and fluorine-19 detection. NMR Biomed 26:860–871
Bloembergen N, Morgan LO (1961) Proton relaxation times in paramagnetic solutions. Effects of electron spin relaxation. J Chem Phys 34:842–850
Kislukhin AA, Xu H, Adams SR, Narsinh KH, Tsien RY, Ahrens ET (2016) Paramagnetic fluorinated nanoemulsions for sensitive cellular fluorine-19 magnetic resonance imaging. Nat Mater 15:662–668
Pelura T, Johnson C, Tarara T, Weers J (1992) Stabilization of perflubron emulsions with egg yolk phospholipid. Biomat Artif Cell Immobil 20:845–848
Rossi M (2007) Use of lecithin and lecithin fractions. In: Huopalahti R, López-Fandiño R, Anton M, Schade R (eds) Bioactive egg compounds. Springer Berlin Heidelberg, Berlin, pp 229–239
Grapentin C, Temme S, Mayenfels F et al (2014) Optimization of perfluorocarbon nanoemulsions for molecular imaging by 19F MRI. In: Seifalian A, de Mel A, Kalaskar D (eds) Nanomedicine, vol 2014. One Central Press, pp 268–286
Minotti G, Aust S (1987) The requirement for iron(III) in the initiation of lipid peroxidation by iron(II) and hydrogen peroxide. J Biol Chem 262:1098–1104
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Phys 37:911–917
Ichihara K, Fukubayashi Y (2010) Preparation of fatty acid methyl esters for gas-liquid chromatography. J Lipid Res 51:635–640
Janjic J, Ahrens ET (2012) Compositions and methods for producing cellular labels for nuclear magnetic resonance techniques. United States Patent and Trade Mark Office US8227610B2
Postel M, Riess JG, Weers JG (1994) Fluorocarbon emulsions - the stability issue. Artif Cell Blood Subtit 22:991–1005
Riess JG, Postel M (1992) Stability and stabilization of fluorocarbon emulsions destined for injection. Biomat Artif Cell Immobil 20:819–830
Kabalnov AS, Shchukin ED (1992) Ostwald ripening theory: applications to fluorocarbon emulsion stability. Adv Colloid Interf 38:69–97
Johnson DR, Decker EA (2015) The role of oxygen in lipid oxidation reactions: a review. Annu Rev Food Technol 6:171–190
Jacoby C, Temme S, Mayenfels F, Benoit N, Krafft MP, Schubert R, Schrader J, Flögel U (2014) Probing different perfluorocarbons for in vivo inflammation imaging by 19F MRI: image reconstruction, biological half-lives and sensitivity. NMR Biomed 27:261–271
Temme S, Jacoby C, Ding Z, Bonner F, Borg N, Schrader J, Flogel U (2014) Technical advance: monitoring the trafficking of neutrophil granulocytes and monocytes during the course of tissue inflammation by noninvasive 19F MRI. J Leukoc Biol 95:689–697
Jahromi AH, Wang C, Adams SR, Zhu W, Narsinh K, Xu H, Gray DL, Tsien RY, Ahrens ET (2019) Fluorous-soluble metal chelate for sensitive fluorine-19 magnetic resonance imaging nanoemulsion probes. ACS Nano 13:143–151
Ahrens ET, Young W-B, Xu H, Pusateri LK (2011) Rapid quantification of inflammation in tissue samples using perfluorocarbon emulsion and fluorine-19 nuclear magnetic resonance. Biotechniques 50:229–234
Spahn DR (1999) Blood substitutes. Artificial oxygen carriers: perfluorocarbon emulsions. Crit Care 3:R93–R97
Spence R, Norcross E, Costabile J et al (1994) Perfluorocarbons as blood substitutes: the early years: experience with fluosol DA-20% in the 1980s. Artif Cell Blood Subtit 22:955–963
Tremper KK, Vercellotti GM, Hammerschmidt DE (1984) Hemodynamic profile of adverse clinical reactions to fluosol-DA 20%. Crit Care Med 12:428–431
Palacios LE, Wang T (2005) Egg-yolk lipid fractionation and lecithin characterization. J Am Oil Chem Soc 82:571–578
Morrill GA, Kostellow A, Resnick LM, Gupta RK (2004) Interaction between ferric ions, phospholipid hydroperoxides, and the lipid phosphate moiety at physiological pH. Lipids 39:881–889
Halliwell B, Gutteridge JMC (1990) Role of free-radicals and catalytic metal-ions in man disease - an overview. Methods Enzymol 186:1–85
Funding
Funding for ETA was provided by National Institutes of Health (NIH) grants R01-EB017271, R01-EB024015, R01-CA134633, and the California Institute for Regenerative Medicine LA1-C12-06919. JR received funding from NIH T32-EB005970.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animal experiments were performed in accordance with the guidelines provided by the UCSD Institutional Animal Care and Use Committee (IACUC) and the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Conflict of Interest
ETA is a founder, consultant, member of the advisory board and shareholder of Celsense, Inc. The other authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article was updated to correct the Funding Information.
Rights and permissions
About this article
Cite this article
Rho, J., Stares, E., Adams, S.R. et al. Paramagnetic Fluorinated Nanoemulsions for in vivo F-19 MRI. Mol Imaging Biol 22, 665–674 (2020). https://doi.org/10.1007/s11307-019-01415-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-019-01415-5