Abstract
Purpose
Epileptogenesis, i.e., development of epilepsy, involves a number of processes that alter the brain function in the way that triggers spontaneous seizures. Kindling is one of the most used animal models of temporal lobe epilepsy (TLE) and epileptogenesis, although chemical kindling suffers from high inter-assay success unpredictability. This study was aimed to analyze the eventual regional brain metabolic changes during epileptogenesis in the pentylenetetrazole (PTZ) kindling model in order to obtain a predictive kindling outcome parameter.
Procedures
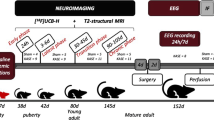
In vivo longitudinal positron emission tomography (PET) scans with 2-deoxy-2-[18F]fluoro-D-glucose ([18F]FDG) along the PTZ kindling protocol (35 mg/kg intraperitoneally (i.p.), 18 sessions) in adult male rats were performed in order to evaluate the regional brain metabolism.
Results
The half of the PTZ-injected rats reached the kindled state. In addition, a significant decrease of [18F]FDG uptake at the end of the protocol in most of the brain structures of kindled animals was found, reflecting the characteristic epilepsy-associated hypometabolism. However, PTZ-injected animals but not reaching the kindled state did not show this widespread brain hypometabolism. Retrospective analysis of the data revealed that hippocampal [18F]FDG uptake normalized to pons turned out to be a predictive index of the kindling outcome. Thus, a 19.06 % reduction (p = 0.008) of the above parameter was found in positively kindled rats compared to non-kindled ones just after the fifth PTZ session.
Conclusion
Non-invasive PET neuroimaging was a useful tool for discerning epileptogenesis progression in this animal model. Particularly, the [18F]FDG uptake of the hippocampus proved to be an early predictive parameter to differentiate resistant and non-resistant animals to the PTZ kindling.




Similar content being viewed by others
References
Akhlaghi Z, Sayyah M, Mokhtari M, Ahmadi A (2012) Effect of intra-amygdala injection of lipopolysaccharide on kindling epileptogenesis in adult rats. Arch Iran Med 15:557–559
Engel JJ (2001) Intractable epilepsy: definition and neurobiology. Epilepsia 42(Suppl 6):3
Goffin K, Dedeurwaerdere S, Van Laere K, Van Paesschen W (2008) Neuronuclear assessment of patients with epilepsy. Semin Nucl Med 38:227–239
Stables JP, Bertram EH, White HS et al (2002) Models for epilepsy and epileptogenesis: report from the NIH workshop, Bethesda, Maryland. Epilepsia 43:1410–1420
Löscher W (2002) Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res 50:105–123
Dedeurwaerdere S, Jupp B, O’Brien TJ (2007) Positron emission tomography in basic epilepsy research: a view of the epileptic brain. Epilepsia 48:56–64
White HS (2002) Animal models of epileptogenesis. Neurology 59:S7–S14
Martín E, Pozo M (2006) Animal models for the development of new neuropharmacological therapeutics in the status epilepticus. Curr Neuropharmacol 4:33–40
Goddard GV, McIntyre DC, Leech CK (1969) A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol 25:295–330
McIntyre DC (2006) The kindling phenomenon. In: Pitkänen A, Schwartzkroin P, Moshe SL (eds) Model. Seizures epilepsy. Elsevier Inc, Amsterdam, pp 351–363
Dhir A (2012) Pentylenetetrazol (PTZ) kindling model of epilepsy. Curr Protoc Neurosci 1:1–12
Ono J, Vieth RF, Walson PD (1990) Electrocorticographical observation of seizures induced by pentylenetetrazol (PTZ) injection in rats. Funct Neurol 5:345–352
Gilbert M, Goodman J (2006) Chemical kindling. In: Pitkänen A, Schwartzkroin P, Moshé S (eds) Model. Seizures epilepsy. Elsevier Inc, Amsterdam, pp 379–391
Corda MG, Orlandi M, Lecca D et al (1991) Pentylenetetrazol-induced kindling in rats: effect of GABA function inhibitors. Pharmacol Biochem Behav 40:329–333
Ito T, Hori M, Yoshida K, Shimizu M (1977) Effect of anticonvulsants on seizures developing in the course of daily administration of pentetrazol to rats. Eur J Pharmacol 45:165–172
O’Brien TJ, Miles K, Ware R et al (2008) The cost-effective use of 18F-FDG PET in the presurgical evaluation of medically refractory focal epilepsy. J Nucl Med 49:931–937
Duncan J (2009) The current status of neuroimaging for epilepsy. Curr Opin Neurol 22:179–184
Goffin K, Van Paesschen W, Dupont P, Van Laere K (2009) Longitudinal microPET imaging of brain glucose metabolism in rat lithium-pilocarpine model of epilepsy. Exp Neurol 217:205–209
Guo Y, Gao F, Wang S et al (2009) In vivo mapping of temporospatial changes in glucose utilization in rat brain during epileptogenesis: an 18F-fluorodeoxyglucose-small animal positron emission tomography study. Neuroscience 162:972–979
Zhang L, Guo Y, Hu H et al (2015) FDG-PET and NeuN-GFAP immunohistochemistry of hippocampus at different phases of the pilocarpine model of temporal lobe epilepsy. Int J Med Sci 12:288–294
Jupp B, Williams J, Binns D et al (2007) Imaging small animal models of epileptogenesis. Neurol Asia 12:51–54
Lee EM, Park GY, Im KC et al (2012) Changes in glucose metabolism and metabolites during the epileptogenic process in the lithium-pilocarpine model of epilepsy. Epilepsia 53:860–869
Shiha AA, de Cristóbal J, Delgado M et al (2015) Subacute administration of fluoxetine prevents short-term brain hypometabolism and reduces brain damage markers induced by the lithium-pilocarpine model of epilepsy in rats. Brain Res Bull 111:36–47
Carmody S, Brennan L (2010) Effects of pentylenetetrazole-induced seizures on metabolomic profiles of rat brain. Neurochem Int 56:340–344
Diehl RG, Smialowski A, Gotwo T (1984) Development and persistence of kindled seizures after repeated injections of pentylenetetrazol in rats and guinea pigs. Epilepsia 25:506–510
Prieto E, Collantes M, Delgado M et al (2011) Statistical parametric maps of 18F-FDG PET and 3-D autoradiography in the rat brain: a cross-validation study. Eur J Nucl Med Mol Imaging 38:2228–2237
Schiffer WK, Mirrione MM, Biegon A et al (2006) Serial microPET measures of the metabolic reaction to a microdialysis probe implant. J Neurosci Methods 155:272–284
Park GY, Lee EM, Seo M-S et al (2015) Preserved hippocampal glucose metabolism on (18)F-FDG PET after transplantation of human umbilical cord blood-derived mesenchymal stem cells in chronic epileptic rats. J Korean Med Sci 30:1232–1240
Ono J, Walson PD (1991) Effects of injection interval on pentylenetetrazol (PTZ) kindled seizures in rats. Med J Osaka Univ 40:45–49
Fischer W, Kittner H (1998) Influence of ethanol on the pentylenetetrazol-induced kindling in rats. J Neural Transm 105:1129–1142
Fang F, Lei H (2010) Increased hippocampal T2 in a rat model of pentylenetetrazol-induced kindling correlates with seizure scores. J Neurol Sci 292:16–23
Kaya M, Gurses C, Kalayci R et al (2008) Morphological and functional changes of blood–brain barrier in kindled rats with cortical dysplasia. Brain Res 1208:181–191
Vivash L, Gregoire M-C, Lau EW et al (2013) 18F-flumazenil: a γ-aminobutyric acid A-specific PET radiotracer for the localization of drug-resistant temporal lobe epilepsy. J Nucl Med 54:1270–1277
Abadie P, Baron JC, Bisserbe JC et al (1992) Central benzodiazepine receptors in human brain: estimation of regional B max and KD values with positron emission tomography. Eur J Pharmacol 213:107–115
Abadie P, Baron JC, Bisserbe JC et al (1991) The central benzodiazepine receptor (cBZR): studies by PET. Eur Neuropsychopharmacol 1:226–229
Millet P, Graf C, Buck A et al (2002) Evaluation of the reference tissue models for PET and SPECT benzodiazepine binding parameters. Neuroimage 17:928–942
Delforge J, Pappata S, Millet P et al (1995) Quantification of benzodiazepine receptors in human brain using PET, [11C]flumazenil, and a single-experiment protocol. J Cereb Blood Flow Metab 15:284–300
Jupp B, Williams J, Binns D et al (2012) Hypometabolism precedes limbic atrophy and spontaneous recurrent seizures in a rat model of TLE. Epilepsia 53:1233–1244
Virdee K, Cumming P, Caprioli D et al (2012) Applications of positron emission tomography in animal models of neurological and neuropsychiatric disorders. Neurosci Biobehav Rev 36:1188–1216
Keogh BP, Cordes D, Stanberry L et al (2005) BOLD-fMRI of PTZ-induced seizures in rats. Epilepsy Res 66:75–90
Choy M, Dubé CM, Patterson K et al (2014) A novel, noninvasive, predictive epilepsy biomarker with clinical potential. J Neurosci 34:8672–8684
Jin Y, Lim C-M, Kim S-W et al (2009) Fluoxetine attenuates kainic acid-induced neuronal cell death in the mouse hippocampus. Brain Res 1281:108–116
Baracskay P, Szepesi Z, Orbán G et al (2008) Generalization of seizures parallels the formation of “dark” neurons in the hippocampus and pontine reticular formation after focal-cortical application of 4-aminopyridine (4-AP) in the rat. Brain Res 1228:217–228
Duveau V, Arthaud S, Serre H et al (2005) Transient hyperthermia protects against subsequent seizures and epilepsy-induced cell damage in the rat. Neurobiol Dis 19:142–149
O’Brien TJ, Newton MR, Cook MJ et al (1997) Hippocampal atrophy is not a major determinant of regional hypometabolism in temporal lobe epilepsy. Epilepsia 38:74–80
Fink GR, Pawlik G, Stefan H et al (1996) Temporal lobe epilepsy: evidence for interictal uncoupling of blood flow and glucose metabolism in temporomesial structures. J Neurol Sci 137:28–34
Foldvary N, Lee N, Hanson MW et al (1999) Correlation of hippocampal neuronal density and FDG-PET in mesial temporal lobe epilepsy. Epilepsia 40:26–29
Garcia-Garcia L, Shiha AA, Bascunana P et al (2015) Serotonin depletion does not modify the short-term brain hypometabolism and hippocampal neurodegeneration induced by the lithium-pilocarpine model of status epilepticus in rats. Cell Mol Neurobiol. doi:10.1007/s10571-015-0240-4
Acknowledgments
This work was funded by the Spanish Ministry of Science (SAF2009-09020) and Comunidad de Madrid grants (I2M2; P2010/BMD-2349). Pablo Bascuñana was financially supported by the “Alfonso Casanava” predoctoral research grant (Instituto Tecnológico PET-Universidad Complutense de Madrid).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All the procedures were performed in accordance with the guidelines of the European Union (2010/63/EU) for the use of animals for scientific purposes. This study was approved by the Ethical Animal Research Committee of the Universidad Complutense de Madrid. All efforts were made to minimize suffering and the number of animals used in this study.
Conflict of Interest
The authors declare that they have no conflict interest.
Rights and permissions
About this article
Cite this article
Bascuñana, P., Javela, J., Delgado, M. et al. [18F]FDG PET Neuroimaging Predicts Pentylenetetrazole (PTZ) Kindling Outcome in Rats. Mol Imaging Biol 18, 733–740 (2016). https://doi.org/10.1007/s11307-016-0950-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-016-0950-0