Abstract
Purpose
[18F]fluorodeoxysorbitol ([18F]FDS) is the first radiopharmaceutical specific for a category of bacteria and has the potential to specifically detect Enterobacteriaceae infections. The purpose of this study was to testify the safety and investigate the biodistribution and radiation dosimetry of [18F]FDS in healthy human bodies.
Procedures
Six healthy subjects were intravenously injected with 320–520 MBq [18F]FDS. On each subject, 21 whole-body emission scans and a brain scan were conducted at settled time points within the next 4 h. Residence time for each source organ was determined by multi-exponential regression. Absorbed doses for target organs and effective dose were calculated via OLINDA/EXM.
Results
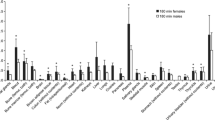
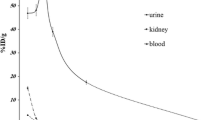
No adverse events due to [18F]FDS injection were observed in the study. The tracer was cleared rapidly from the blood pool through the urinary system. A small portion was cleared into the gut through the hepatobiliary system. The effective dose (ED) was estimated to be 0.021 ± 0.001 mSv/MBq. The organ receiving the highest absorbed dose was the urinary bladder wall (0.25 ± 0.03 mSv/MBq).
Conclusions
[18F]FDS is safe and well tolerated. The effective dose was comparable to that of other F-18 labeled radiotracers. [18F]FDS is suitable for human use from a radiation dosimetry perspective.



Similar content being viewed by others
References
Wang X, Murthy N (2014) Bacterial imaging comes of age. Sci Transl Med 6:259fs43–259fs43
Littenberg RL, Taketa RM, Alazraki NP et al (1973) Gallium-67 for localization of septic lesions. Ann Intern Med 79:403–406
Erba PA, Martina S, Umberto C et al (2013) Radiolabeled wbc scintigraphy in the diagnostic workup of patients with suspected device-related infections. Jacc Cardiovasc Imaging 6:1075–1086
Pakos EE, Koumoulis HD, Fotopoulos AD et al (2007) Osteomyelitis: antigranulocyte scintigraphy with 99mTc radiolabeled monoclonal antibodies for diagnosis. Radiology 245:732–741
Bleeker-Rovers CP, Rennen HJJM, Boerman OC et al (2007) 99mTc-labeled interleukin 8 for the scintigraphic detection of infection and inflammation: first clinical evaluation. J Nucl Med 48:337–343
Qiu L, Chen Y (2012) The role of 18F-FDG PET or PET/CT in the detection of fever of unknown origin. Eur J Radiol 81:3524–9
Nibbering PH, Welling MM, Paulusma-Annema A et al (2004) 99mTc-labeled UBI 29-41 peptide for monitoring the efficacy of antibacterial agents in mice infected with staphylococcus aureus. J Nucl Med 45:321–326
Petrik M, Haas H, Laverman P et al (2014) 68Ga-Triacetylfusarinine C and 68Ga-Ferrioxamine E for Aspergillus infection imaging: uptake specificity in various microorganisms. Mol Imaging Biol 16:102–108
Siaens R, Eijsink VG, Dierckx R et al (2004) 123I-labeled chitinase as specific radioligand for in vivo detection of fungal infections in mice. J Nucl Med 45:1209–1216
Diaz LA, Foss CA, Thornton K et al (2007) Imaging of musculoskeletal bacterial infections by [124I]FIAU-PET/CT. PLoS ONE 2:e1007
Gowrishankar G, Namavari M, Jouannot EB et al (2014) Investigation of 6-[18F]-Fluoromaltose as a novel PET tracer for imaging bacterial infection. Plos One 9:e107951–e107951
Xinghai N, Wonewoo S, Seungjun L et al (2014) PET imaging of bacterial infections with fluorine-18-labeled maltohexaose. Angew Chem Int Ed 53:14096–14101
Weinstein EA, Ordonez AA, Demarco VP et al (2014) Imaging Enterobacteriaceae infection in vivo with 18F-fluorodeoxysorbitol positron emission tomography. Sci Transl Med 6:259ra146–259ra146
Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. National Cancer Institute. Published on June 14, 2010. www.ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40
Tang GH, Tang XL, Wang MF et al (2006) High efficient automated synthesis of 2-[18F] fluoro-2-deoxy-D-glucose. Nucl Technol 29:531–536
Li ZB, Wu Z, Cao Q et al (2008) The synthesis of 18F-FDS and its potential application in molecular imaging. Mol Imaging Biol 10:92–98
Wolf I, Vetter M, Wegner I et al (2005) The medical imaging interaction toolkit. Med Image Anal 9:594–604
Stabin MG, Sparks RB, Crowe E (2005) OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med 46:1023–1027
International Commission on Radiological Protection (ICRP) (1994) Basic anatomical and physiological data for use in radiological protection: the skeleton. ICRP publication 70. Ann ICRP 25:66–67
Yao SB, Xing HQ, Zhu WJ et al (2016) Infection imaging with 18F-FDS and first-in-human evaluation, Nucl Med Biol 43:206–14
International Commission on Radiological Protection (ICRP) (2015) Radiation dose to patients from radiopharmaceuticals: a compendium of current information related to frequently used substances. ICRP Publication 128. Ann ICRP 44:107–109
Radioactive drugs for certain research uses. Code of Federal Regulations. Title 21, Volume 5. Sec. 361.1. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr5361
Kaushik A, Jaimini A, Tripathi M et al (2015) Estimation of radiation dose to patients from (18) FDG whole body PET/CT investigations using dynamic PET scan protocol. Indian J Med Res 142:721–31
Divoli A, Chiavassa S, Ferrer L et al (2009) Effect of patient morphology on dosimetric calculations for internal irradiation as assessed by comparisons of Monte Carlo versus conventional methodologies. J Nucl Med 50:316–323
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (Grant No. 81071188 and 81571713). We would like to extend our heartiest gratitude to Prof. Yun Zhou for the support of data management and study preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures of the study were approved by the Institutional Review Board of Peking Union Medical College Hospital.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhu, W., Yao, S., Xing, H. et al. Biodistribution and Radiation Dosimetry of the Enterobacteriaceae-Specific Imaging Probe [18F]Fluorodeoxysorbitol Determined by PET/CT in Healthy Human Volunteers. Mol Imaging Biol 18, 782–787 (2016). https://doi.org/10.1007/s11307-016-0946-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-016-0946-9