Abstract
Purpose
This study is aimed at demonstrating the in vivo potential of Gd(III)-loaded glucan particles (Gd-GPs) as magnetic resonance imaging (MRI)-positive agents for labeling and tracking phagocytic cells.
Procedure
GPs were obtained from Saccharomyces cerevisae and loaded with the water-insoluble complex Gd-DOTAMA(C18)2. The uptake kinetics of Gd-GPs by murine macrophages was studied in vitro and the internalization mechanism was assessed by competition assays. The in vivo performance of Gd-GPs was tested at 7.05 T on a mouse model of acute liver inflammation.
Results
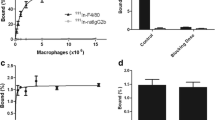
The minimum number of Gd-GPs-labeled J774.A1 macrophages detected in vitro by MRI was ca. 300 cells/μl of agar, which is the lowest number ever reported for cells labeled with a positive T1 agent. Intravenous injection of macrophages labeled with Gd-GPs in a mouse model of liver inflammation enabled the MRI visualization of the cellular infiltration in the diseased area.
Conclusions
Gd-GPs represent a promising platform for tracking macrophages by MRI as a T1 alternative to the golden standard T2-based iron oxide particles.





Similar content being viewed by others
Abbreviations
- Gd-GPs:
-
Gd-loaded glucan particles
- GPs:
-
Glucan particles
- Rho:
-
GPs rhodamine-loaded glucan particles
- PBS:
-
Phosphate-buffered saline
- ALT:
-
Alanine amino transferase
- USPIO:
-
Ultra-small superparamagnetic iron oxide particles
- SPIO:
-
Superparamagnetic iron oxide particles
- MPIO:
-
Micrometer-sized iron oxide particles
- siRNA:
-
Small interfering RNA
References
Lu CW, Hung Y, Hsiao JK et al (2007) Bifunctional magnetic silica nanoparticles for highly efficient human stem cell labeling. Nano Lett 7:149–154
Shapiro EM, Gonzalez-Perez O, Manuel Garcia-Verdugo J et al (2006) Magnetic resonance imaging of the migration of neuronal precursors generated in the adult rodent brain. NeuroImage 32:1150–1157
Arbab AS, Yocum GT, Kalish H et al (2004) Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood 104:1217–1223
Tang KS, Shapiro EM (2011) Enhanced magnetic cell labeling efficiency using –NH2 coated MPIOs. Magn Reson Med 65:1564–1569
Giesel FL, Stroick M, Griebe M et al (2006) Gadofluorine uptake in stem cells as a new magnetic resonance imaging tracking method: an in vitro and in vivo study. Invest Radiol 41:868–873
Geninatti Crich S, Biancone L, Cantaluppi V et al (2004) Improved route for the visualization of stem cells labeled with a Gd-/Eu-chelate as dual (MRI and fluorescence) agent. Magn Reson Med 51:938–944
Biancone L, Geninatti Crich S, Cantaluppi V et al (2007) Magnetic resonance imaging of gadolinium-labeled pancreatic islets for experimental transplantation. NMR Biomed 20:40–48
Menchise V, Digilio G, Gianolio E et al (2011) In vivo labeling of B16 melanoma tumor xenograft with a thiol-reactive gadolinium based MRI contrast agent. Mol Pharm 8:1750–1756
Hedlund A, Ahrén M, Gustafsson H et al (2011) Gd2O3 nanoparticles in hematopoietic cells for MRI contrast enhancement. Int J Nanomedicine 6:3233–3240
Guenoun J, Koning GA, Doeswijk G et al (2012) Cationic Gd-DTPA liposomes for highly efficient labeling of mesenchymal stem cells and cell tracking with MRI. Cell Transplant 21:191–205
Ribot EJ, Miraux S, Konsman JP et al (2011) In vivo MR tracking of therapeutic microglia to a human glioma model. NMR Biomed 24:1361–1368
Tachibana Y, Enmi J, Mahara A et al (2010) Design and characterization of a polymeric MRI contrast agent based on PVA for in vivo living-cell tracking. Contrast Media Mol Imaging 5:309–317
Tran LA, Krishnamurthy R, Muthupillai R et al (2010) Gadonanotubes as magnetic nanolabels for stem cell detection. Biomaterials 31:9482–9491
Tseng CL, Shih IL, Stobinski L, Lin FH (2010) Gadolinium hexanedione nanoparticles for stem cell labeling and tracking via magnetic resonance imaging. Biomaterials 31:5427–5435
Fizet J, Rivière C, Bridot JL et al (2009) Multi-luminescent hybrid gadolinium oxide nanoparticles as potential cell labeling. J Nanosci Nanotechnol 9:5717–5725
Nolte IS, Gungor S, Erber R et al (2008) In vitro labeling of glioma cells with gadofluorine M enhances T1 visibility without affecting glioma cell growth or motility. Magn Reson Med 59:1014–1020
New EJ, Parker D, Smith DG, Walton JW (2010) Development of responsive lanthanide probes for cellular applications. Cur Opinion Chem Biol 14:238–246
Bulte JW, Kraitchman DL (2004) Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed 17:484–499
Ahrens ET, Flores R, Xu H, Morel PA (2005) In vivo imaging platform for tracking immunotherapeutic cells. Nat Biotechnol 23:983–987
Ottobrini L, Martelli C, Trabattoni DL et al (2011) In vivo imaging of immune cell trafficking in cancer. Eur J Nucl Med Mol Imaging 38:949–968
Aouadi M, Tesz GJ, Nicoloro SM et al (2009) Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature 458:1180–1184
Soto ER, Caras AC, Kut LC, et al. (2012) Glucan particles for macrophage targeted delivery of nanoparticles. J Drug Deliv (in press)
Soto E, Kim YS, Lee J et al (2010) Glucan particle encapsulated rifampicin for targeted delivery to macrophages. Polymers 2:681–689
Soto ER, Ostroff GR (2008) Characterization of multilayered nanoparticles inside yeast cell wall particles for DNA delivery. Bioconjugate Chem 19:840–848
Tesz GJ, Aouadi M, Prot M et al (2011) Glucan particles for selective delivery of siRNA to phagocytic cells in mice. Biochem J 436:351–362
Soto E, Ostroff G (2011) Use of beta-1,3-d-glucans for drug delivery applications. In: Vetvicka V, Novak M (eds) Biology and chemistry of beta glucan (volume 1): beta glucans-mechanisms of action. Bentham Press, Oak Park, p 82
Figueiredo S, Moreira JN, Geraldes CF et al (2011) Yeast cell wall particles: a promising class of nature-inspired microcarriers for multimodal imaging. Chem Commun 47:10635–10637
Brown GD, Gordon S (2001) Immune recognition: a new receptor for β-glucans. Nature 413:36–37
Chan GC, Chan WK, Sze DM (2009) The effects of beta-glucan on human immune and cancer cells. J Hematol Oncol 2:25–35
Huang H, Ostroff GR, Lee CK et al (2009) Distinct patterns of dendritic cell cytokine release stimulated by fungal beta-glucans and toll-like receptor agonists. Infect Immun 77:1774–1781
Huang H, Ostroff GR, Lee CK et al (2012) Relative contributions of Dectin-1 and complement to immune responses to particulate β-glucans. J Immunol 189:312–317
Vu-Quang H, Muthiah M, Lee HJ et al (2012) Immune cell-specific delivery of beta-glucan-coated iron oxide nanoparticles for diagnosing liver metastasis by MR imaging. Carbohydr Polym 87:1159–1168
Anelli PL, Lattuada L, Lorusso V et al (2001) Mixed micelles containing lipophilic gadolinium complexes as MRA contrast agents. Magn Reson Mater Phys Biol Med 12:114–120
Gianolio E, Arena F, Strijkers GJ et al (2011) Photochemical activation of endosomal escape of MRI-Gd-agents in tumor cells. Magn Reson Med 65:212–219
Domenicali M, Caraceni P, Giannone F et al (2009) A novel model of CCl4-induced cirrhosis with ascites in the mouse. J Hepatol 51:991–999
Ramachandran R, Kakar S (2009) Histological patterns in drug-induced liver disease. J Clin Pathol 62:481–492
Becker A, Neumeier R, Heidrich C et al (1986) Cell surface glycoproteins of hepatocytes and hepatoma cells identified by monoclonal antibodies. Biol Chem Hoppe-Seyler 367:681–688
Rejman J, Oberle V, Zuhorn IS, Hoekstra D (2004) Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J 377:159–169
Terreno E, Delli Castelli D, Viale A, Aime S (2010) Challenges for molecular magnetic resonance imaging. Chem Rev 110:3019–3042
Terreno E, Geninatti Crich S, Belfiore S et al (2006) Effect of the intracellular localization of a Gd-based imaging probe on the relaxation enhancement of water protons. Magn Reson Med 55:491–497
Huang H, Ostroff GR, Lee CK et al (2011) Robust stimulation of humoral and cellular immune responses following vaccination with antigen-loaded β-glucan particles. MBio 2:e00164–10
Goodridge HS, Underhill DM, Touret N (2012) Mechanisms of Fc receptor and Dectin-1 activation for phagocytosis. Traffic 13:1062–1071
Schneider B, Schueller C, Utermoehlen O, Haas A (2007) Lipid microdomain-dependent macropinocytosis determines compartmentation of Afipia felis. Traffic 8:226–240
Scherbart AM, Langer J, Bushmelev A et al (2011) Contrasting macrophage activation by fine and ultrafine titanium dioxide particles is associated with different uptake mechanisms. Part Fibre Toxicol 8, art.31
Lim JP, Gleeson PA (2011) Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol Cell Biol 89:836–843
Fernando LP, Kandel PK, Yu J et al (2010) Mechanism of cellular uptake of highly fluorescent conjugated polymer nanoparticles. Biomacromolecules 11:2675–2682
Karlmark KR, Weiskirchen R, Zimmermann HW et al (2009) Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology 50:261–274
Tajima T, Murata T, Aritake K et al (2008) Lipopolysaccharide induces macrophage migration via prostaglandin D(2) and prostaglandin E(2). J Pharmacol Exp Ther 326:493–501
Herre J, Marshall AS, Caron E et al (2004) Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages. Blood 104:4038–4045
Akramiene D, Kondrotas A, Didziapetriene J, Kevelaitis E (2007) Effects of β-glucans on the immune system. Medicina (Kaunas) 43:597–606
Nestle FO, Banchereau J, Hart D (2001) Dendritic cells: on the move from bench to bedside. Nat Med 7:761–765
Morel PA, Feili-Hariri M, Coates PT, Thomson AW (2003) Dendritic cells, T cell tolerance and therapy of adverse immune reactions. Clin Exp Immunol 133:1–10
Acknowledgments
The authors thank Regione Piemonte (PIIMDMT and nano-IGT projects), Foundation for Science and Technology of Portugal (project PTDC/QUI/70063/2006, contract REDE/1517/RMN/2005, and grant No. SFRH/BD/33187/2007 to SF) and FEDER, for their support. This work was carried out in the framework of the EU COST D38 and TD1004 Actions and EU-FP7 projects ENCITE (grant No. 201842) and INMiND (grant No. 278850).
Conflict of Interest
Dr. Terreno and Dr. Aime are consultants for Bracco Imaging S.p.A. The other authors have no potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 13844 kb)
Rights and permissions
About this article
Cite this article
Figueiredo, S., Cutrin, J.C., Rizzitelli, S. et al. MRI Tracking of Macrophages Labeled with Glucan Particles Entrapping a Water Insoluble Paramagnetic Gd-Based Agent. Mol Imaging Biol 15, 307–315 (2013). https://doi.org/10.1007/s11307-012-0603-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-012-0603-x