Abstract
Purpose
The chick embryo is a well-known economical in vivo model system and is widely applied in preclinical research, e.g., bone development studies. It is therefore surprising that no studies concerning the application of 18F-fluoride microPET to bone metabolism have been reported so far. This may be due to motion artifacts or the lack of convenient tracer injection sites.
Methods
We resolved the above problems using a combination of sample preparation, anesthesia, microPET imaging, and computational processing, and describe a convenient way of visualizing three- and four- dimensional features of bone metabolism in living chick embryos.
Results
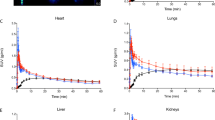
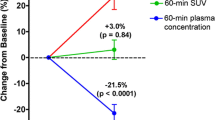
The application of 18F-fluoride microPET facilitates repeat measurements, highly reproducible and motion-artifact-free skeletal imaging, and provides quantitative measurements of in ovo metabolic activities in the bones of developing chick. During microPET measurement, radio tracer was injected intravascularly using a custom-made catheter system, allowing us to additionally investigate early time points in tracer kinetics and uptake.
Conclusions
Our results show that bone metabolism in living chick embryos can be reproducibly studied and quantified in ovo, even for multiple tracer injections over a longer time period. The use of dynamic 18F-fluoride microPET imaging made it possible to visualize and analyze even small bone structures with excellent quality. Moreover, as our data are comparable to data from corresponding rodent experiments, the use of embryonated chicken eggs is a convenient and economical alternative to other animal models.






Similar content being viewed by others
References
Rashidi H, Sottile V (2009) The chick embryo: hatching a model for contemporary biomedical research. Bioessays 31(4):459–465
Darnell DK, Schoenwolf GC (1999) The chick embryo as a model system for analyzing mechanisms of development. Methods Mol Biol 135:25–29
Deryugina EI, Quigley JP (2008) Chick embryo chorioallantoic membrane model systems to study and visualize human tumor cell metastasis. Histochem Cell Biol 130(6):1119–1130
Glover JC, Boulland JL, Halasi G, Kasumacic N (2009) Chimeric animal models in human stem cell biology. ILAR J 51(1):62–73
Härtl A, Sauerbrei A, Stelzner A, Wutzler P (2004) Influenza infection of the embryonated hen's egg/an alternative model for in vivo evaluation of antiviral compounds. Arzneimittelforschung 54(2):130–134
Zhu L, van de Lavoir M-C, Albanese J, Beenhouwer DO, Cardarelli PM, Cuison S et al (2005) Production of human monoclonal antibody in eggs of chimeric chickens. Nat Biotechnol 23(9):1159–1169
Stern CD (2005) The chick: a great model system becomes even greater. Dev Cell 8(1):9–17
Cook ME (2000) Skeletal deformities and their causes: introduction. Poult Sci 79(7):982–984
Brugmann SA, Powder KE, Young NM, Goodnough LH, Hahn SM, James AW et al (2010) Comparative gene expression analysis of avian embryonic facial structures reveals new candidates for human craniofacial disorders. Hum Mol Genet 19(5):920–930
Balke M, Neumann A, Kersting C, Agelopoulos K, Gebert C, Gosheger G et al (2010) Morphologic characterization of osteosarcoma growth on the chick chorioallantoic membrane. BMC Res Notes 3:58
Kulesa PM, Bailey CM, Cooper C, Fraser SE (2010) In ovo live imaging of avian embryos. Cold Spring Harb Protoc. doi:10.1101/pdb.prot5446
Bain MM (2007) Noninvasive monitoring of chick development in ovo using a 7 T MRI system from day 12 of incubation through to hatching. J Magn Reson Imaging 26(1):198–201
Li X, Liu J, Davey M, Duce S, Jaberi N, Liu G et al (2007) Micro-magnetic resonance imaging of avian embryos. J Anat 211(6):798–809
Hogers B, van der Weerd L, Olofsen H, van der Graaf LM, DeRuiter MC, Gittenberger-de Groot AC et al (2008) Non-invasive tracking of avian development in vivo by MRI. NMR Biomed 22(4):365–373
Duce S, Morrison F, Welten M, Baggott G, Tickle C (2011) Micro-magnetic resonance imaging study of live quail embryos during embryonic development. Magn Reson Imaging 29(1):132–139
Heidrich A, Würbach L, Opfermann T, Saluz HP (2011) Motion-artifact-free in vivo imaging utilizing narcotized avian embryos in ovo. Mol Imaging Biol 13(2):208–214
Henning AL, Jiang MX, Yalcin HC, Butcher JT (2011) Quantitative three-dimensional imaging of live avian embryonic morphogenesis via micro-computed tomography. Dev Dyn 240(8):1949–1957
Webb DR (1987) Thermal tolerance of avian embryos: a review. Condor 89(4):874–898
Cook GJ, Lodge Ma, Blake GM, Marsden PK, Fogelman I (2000) Differences in skeletal kinetics between vertebral and humeral bone measured by 18F-fluoride positron emission tomography in postmenopausal women. J Bone Miner Res 15(4):763–769
Blake GM, Park-Holohan SJ, Cook GJ, Fogelman I (2001) Quantitative studies of bone with the use of 18F-fluoride and 99mTc-methylene diphosphonate. Semin Nucl Med 31(1):28–49
Grant FD, Fahey FH, Packard AB, Davis RT, Alavi A, Treves ST (2008) Skeletal PET with 18F-fluoride: applying new technology to an old tracer. J Nucl Med 49(1):68–78
Derlin T, Wisotzki C, Richter U, Apostolova I, Bannas P, Weber C et al (2011) in vivo imaging of mineral deposition in carotid plaque using 18F-sodium fluoride PET/CT: correlation with atherogenic risk factors. J Nucl Med 52(3):362–368
Vaquero JJ, Desco M, Pascau J, Santos A, Seidel J, Green MV (2001) PET, CT, and MR image registration of the rat brain and skull. IEEE Trans Nucl Sci 48(4):1440–1445
Berger F, Lee Y-P, Loening A, Chatziioannou A, Freedland S, Leahy R et al (2002) Whole-body skeletal imaging in mice utilizing microPET: optimization of reproducibility and applications in animal models of bone disease. Eur J Nucl Med Mol Imaging 29(9):1225–1236
Hsu WK, Virk MS, Feeley BT, Stout DB, Chatziioannou AF, Lieberman JR (2008) Characterization of osteolytic, osteoblastic, and mixed lesions in a prostate cancer mouse model using 18F-FDG and 18F-fluoride PET/CT. J Nucl Med 49(3):414–421
Dyke JP, Aaron RK (2010) Noninvasive methods of measuring bone blood perfusion. Ann N Y Acad Sci 1192(1):95–102
Blau M, Ganatra R, Bender MA (1972) 18F-fluoride for bone imaging. Semin Nucl Med 2(1):31–37
Czernin J, Satyamurthy N, Schiepers C (2010) Molecular mechanisms of bone 18F-NaF deposition. J Nucl Med 51(12):1826–1829
Even-Sapir E, Metser U, Flusser G, Zuriel L, Kollender Y, Lerman H et al (2004) Assessment of malignant skeletal disease: initial experience with 18F-fluoride PET/CT and comparison between 18F-fluoride PET and 18F-fluoride PET/CT. J Nucl Med 45(2):272–278
Frost ML, Blake GM, Cook GJR, Marsden PK, Fogelman I (2009) Differences in regional bone perfusion and turnover between lumbar spine and distal humerus: 18F-fluoride PET study of treatment-naïve and treated postmenopausal women. Bone 45(5):942–948
Hawkins RA, Choi Y, Huang SC, Hoh CK, Dahlbom M, Schiepers C et al (1992) Evaluation of the skeletal kinetics of fluorine-18-fluoride ion with PET. J Nucl Med 33(5):633–642
Tazawa H (1981) Measurement of blood pressure of chick embryo with an implanted needle catheter. J Appl Physiol 51(4):1023–1026
Wilson SM, Chambers AF (2004) Experimental metastasis assays in the chick embryo. Curr Protoc Cell Biol 21:19.6.1–19.6.25.
Disselhorst JA, Brom M, Laverman P, Slump CH, Boerman OC, Oyen WJG et al (2010) Image-quality assessment for several positron emitters using the NEMA NU 4-2008 standards in the Siemens Inveon small-animal PET scanner. J Nucl Med 51(4):610–617
Visser EP, Disselhorst JA, Brom M, Laverman P, Gotthardt M, Oyen WJG et al (2009) Spatial resolution and sensitivity of the Inveon small-animal PET scanner. J Nucl Med 50(1):139–147
Wang Y, Dur O, Patrick MJ, Tinney JP, Tobita K, Keller BB et al (2009) Aortic arch morphogenesis and flow modeling in the chick embryo. Ann Biomed Eng 37(6):1069–1081
Doh ST, Hao H, Loh SC, Patel T, Tawil HY, Chen DK et al (2010) Analysis of retinal cell development in chick embryo by immunohistochemistry and in ovo electroporation techniques. BMC Dev Biol 10:8
Shapiro F (1992) Vertebral development of the chick embryo during days 3–19 of incubation. J Morphol 213(3):317–333
Bellairs R, Osmond M (2005) Plate 148-150. In: The atlas of chick development. London: Elsevier Academic Press, pp 406–409
Starck JM (1996) Comparative morphology and cytokinetics of skeletal growth in hatchlings of altricial and precocial birds. Zool Anz 235(1):53–75
Kobayashi H, Kawamoto S, Jo S-K, Sato N, Saga T, Hiraga A et al (2002) Renal tubular damage detected by dynamic micro-MRI with a dendrimer-based magnetic resonance contrast agent. Kidney Int 61(6):1980–1985
Lewis JS, Achilefu S, Garbow JR, Laforest R, Welch MJ (2002) Small animal imaging. current technology and perspectives for oncological imaging. Eur J Cancer 38(16):2173–2188
Azhdarinia A, Ghosh P, Ghosh S, Wilganowski N, Sevick-Muraca EM (2011) Dual-labeling strategies for nuclear and fluorescence molecular imaging: a review and analysis. Mol Imaging Biol (in press)
Chen K, Li Z-B, Wang H, Cai W, Chen X (2008) Dual-modality optical and positron emission tomography imaging of vascular endothelial growth factor receptor on tumor vasculature using quantum dots. Eur J Nucl Med Mol Imaging 35(12):2235–2244
Tufan AC, Lale Satiroglu-Tufan N (2005) The chick embryo chorioallantoic membrane as a model system for the study of tumor angiogenesis, invasion and development of anti-angiogenic agents. Curr Cancer Drug Targets 5(4):249–266
Schmidt G, Figueiredo E, Saatkamp M, Bomm E (2009) Effect of storage period and egg weight on embryo development and incubation results. Rev Bras Cienc Avic 11(1):1–5
Hamburger V, Hamilton H (1951) A series of normal stages in the development of the chick embryo. J Morphol 88(1):49–92
Schellpfeffer M, Bolender DL, Kolesari GL (2007) High frequency ultrasound imaging of the growth and development of the normal chick embryo. Ultrasound Med Biol 33(5):751–761
Acknowledgements
This work was funded by the German Federal Ministry of Education and Health (BMBF; Grant Nos. 0314108 and 01KI1011D).
We are indebted to Brent Sørensen for proofreading the manuscript. We also thank Vera Klujewa for the excellent technical help.
Conflict of Interest Disclosure
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Lydia Würbach and Alexander Heidrich Contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
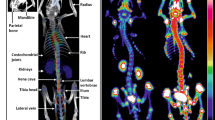
Maximum intensity projections (MIP) of reconstructed microPET data from 55 time frames depicting the application, distribution, and uptake process of 18F-fluoride in the body and bones of a healthy chick embryo in ovo at day 18 of incubation (MPG 990 kb)
ESM 1
(PDF 447 kb)
Rights and permissions
About this article
Cite this article
Würbach, L., Heidrich, A., Opfermann, T. et al. Insights into Bone Metabolism of Avian Embryos In Ovo Via 3D and 4D 18F-fluoride Positron Emission Tomography. Mol Imaging Biol 14, 688–698 (2012). https://doi.org/10.1007/s11307-012-0550-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-012-0550-6