Abstract
Objectives
This study aims to determine the feasibility and utility of functional imaging of copper metabolism imbalance in Atp7b −/− knockout mouse model of Wilson’s disease (WD) with positron emission tomography-computed tomography (PET-CT) using orally administered copper-64 chloride (64CuCl2) as a tracer.
Procedures
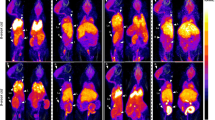
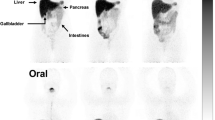
Atp7b −/− KO mice (N = 5) were subjected to PET scanning using a hybrid PET-CT scanner, after oral administration of 64CuCl2 as a tracer. Time-dependent PET quantitative analysis was performed to assess gastrointestinal absorption and biodistribution of 64Cu radioactivity in the Atp7b −/− KO mice, using C57BL wild-type (WT) mice (N = 5) as a normal control. Estimates of human radiation dosimetry were calculated based on biodistribution of 64Cu radioactivity in live animals.
Results
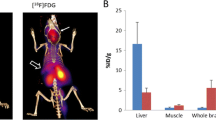
PET-CT analysis demonstrated higher 64Cu radioactivity in the liver of Atp7b −/− knockout mice compared with that in the control C57BL WT mice (p < 0.001), following oral administration of 64CuCl2 as a tracer. In addition, 64Cu radioactivity in the lungs of the Atp7b −/− knockout mice was slightly higher than those in the control C57BL WT mice (p = 0.01). Despite initially higher renal clearance of 64Cu, there was no significant difference of 64Cu radioactivity in the kidneys of the Atp7b −/− KO mice and the control C57BL WT mice at 24 h post-oral administration of 64CuCl2 (p = 0.16). There was no significant difference in low 64Cu radioactivity in the blood, brain, heart, and muscles between the Atp7b −/− knockout mice and control C57BL WT mice (p > 0.05). Based on the biodistribution of 64Cu radioactivity in C57BL WT mice, radiation dosimetry estimates of 64Cu in normal human subjects were obtained. An effective dose (ED) of 42.4 μSv/MBq (weighted dose over 22 organs) was calculated and the lower large intestines were identified as the critical organ for radiation exposure (120 μGy/MBq for males and 135 μGy/MBq for females). Radiation dosimetry estimates for patients with WD, derived from the biodistribution of 64Cu in Atp7b −/− KO mice, showed a slightly lower ED of 37.5 μSv/MBq, with the lower large intestines as the critical organ for radiation exposure (83 μSv/MBq for male and 95 μSv/MBq for female).
Conclusions
PET-CT quantitative analysis demonstrated an increased level of 64Cu radioactivity in the liver of Atp7b −/− KO mice compared with that in the control C57BL WT mice, following oral administration of 64CuCl2 as a tracer. The results of this study suggest the feasibility and utility of PET-CT using orally administered 64CuCl2 as a tracer (64CuCl2-PET/CT) for functional imaging of copper metabolism imbalance in WD.





Similar content being viewed by others
References
Olivares M, Uauy R (1996) Copper as an essential nutrient. Am J Clin Nutr 63(5):791S–796S
Uauy R, Olivares M, Gonzalez M (1998) Essentiality of copper in humans. Am J Clin Nutr 67(5 Suppl):952S–959S
Uriu-Adams JY, Scherr RE, Lanoue L, Keen CL (2010) Influence of copper on early development: prenatal and postnatal considerations. Biofactors 36(2):136–152
Dunlap WM, James GW III, Hume DM (1974) Anemia and neutropenia caused by copper deficiency. Ann Intern Med 80:470–476
Li Y, Wang L, Schuschke DA, Zhou Z, Saari JT, Kang YJ (2005) Marginal dietary copper restriction induces cardiomyopathy in rats. J Nutr 135(9):2130–2136
Kumar N, Gross JB Jr, Ahlskog JE (2003) Myelopathy due to copper deficiency. Neurology 61:273–274
Kumar N, Gross JB Jr, Ahlskog JE (2004) Copper deficiency myelopathy produces a clinical picture like subacute combined degeneration. Neurology 63:33–39
Kumar N (2006) Copper deficiency myelopathy (human swayback). Mayo Clin Proc 81:1371–1384
Prodan CI, Holland NR, Wisdom PJ, Burstein SA, Bottomley SS (2002) CNS demyelination associated with copper deficiency and hyperzincemia. Neurology 59:1453–1456
Tan JC, Burns DL, Jones RH (2006) Severe ataxia, myelopathy, and peripheral neuropathy due to acquired copper deficiency in a patient with history of gastrectomy. J Parenter Enteral Nutr 30(5):446–450
Juhasz-Pocsine K, Rudnicki SA, Archer RL, Harik SI (2007) Neurologic complications of gastric bypass surgery for morbid obesity. Neurology 68(21):1843–1850
Khaleeli Z, Healy DG, Briddon A, Lunn MP, Reilly MM, Land J, Giovannoni G (2010) Copper deficiency as a treatable cause of poor balance. Br Med J 340:c508
Goez HR, Jacob FD, Fealey RD, Patterson MC, Ramaswamy V, Persad R, Johnson ES, Yager JY (2011) An unusual presentation of copper metabolism disorder and a possible connection with Niemann–Pick type C. J Child Neurol 26:518–521
Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW (1993) The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet 5:327–337
Tanzi RE, Petrukhin K, Chernov I, Pellequer JL, Wasco W, Ross B, Romano DM, Parano E, Pavone L, Brzustowicz LM (1993) The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet 5:344–350
Harris ED (2000) Cellular copper transport and metabolism. Annu Rev Nutr 20:291–310
Puig S, Thiele DJ (2002) Molecular mechanisms of copper uptake and distribution. Curr Opin Chem Biol 6:171–180
Lutsenko S (2010) Human copper homeostasis: a network of interconnected pathways. Curr Opin Chem Biol 14:1–7
Cartwright GE, Wintrobe MM (1964) Copper metabolism in normal subjects. Am J Clin Nutr 14:224–232
Turnlund JR (1998) Human whole-body copper metabolism. Am J Clin Nutr 67(5 Suppl):960S–964S
Owen CA Jr (1964) Distribution of copper in the rat. Am J Physiol 207:446–448
Owen CA Jr (1965) Metabolism of radiocopper (Cu64) in the rat. Am J Physiol 209:900–904
Dunn MA, Green MH, Leach RM Jr (1991) Kinetics of copper metabolism in rats: a compartmental model. Am J Physiol 261(1 Pt 1):E115–E125
Turnlund JR, Keyes WR, Erson HL, Acord LL (1989) Copper absorption and retention in young men at three levels of dietary copper using the stable isotope 65Cu. Am J Clin Nutr 49:870–878
Bush JA, Mahoney JP, Markowitz H, Gubler CJ, Cartwright GE, Wintrobe MM (1955) Studies on copper metabolism. XIV. Radioactive copper studies in normal subjects and in patients with hepatolenticular degeneration. J Clin Invest 34(12):1766–1778
Tauxe WN, Goldstein NP, Randall RV, Gross JB (1966) Radiocopper studies in patients with Wilson’s disease and their relatives. Am J Med 41(3):375–380
Vierling JM, Shrager R, Rumble WF, Aamodt R, Berman MD, Jones EA (1978) Incorporation of radiocopper into ceruloplasmin in normal subjects and in patients with primary biliary cirrhosis and Wilson’s disease. Gastroenterology 74(4):652–660
Osborn SB, Szaz KF, Walshe JM (1996) Studies with radioactive copper (64Cu and 67Cu): abdominal scintiscans in patients with Wilson’s disease. Q J Med 38:467–474
Skromne-Kadlubik G, Diaz JF, Celis C (1975) Basal ganglia scans in the human. J Nucl Med 16(8):787–788
Walshe JM, Potter G (1977) The pattern of the whole body distribution of radioactive copper (67Cu, 64Cu) in Wilson’s disease and various control groups. Q J Med 46:445–462
Bissig KD, Honer M, Zimmermann K, Summer KH, Solioz M (2005) Whole animal copper flux assessed by positron emission tomography in the Long–Evans cinnamon rat—a feasibility study. Biometals 18(1):83–88
Peng F, Lutsenko S, Sun X, Muzik O (2011) Positron emission tomography of copper metabolism in the Atp7b −/− knock-out mouse model of Wilson’s disease. Mol Imag Biol. doi:10.1007/s11307-011-0476-4
Huster D, Finegold MJ, Morgan CT, Burkhead JL, Nixon R, Vanderwerf SM, Gilliam CT, Lutsenko S (2006) Consequences of copper accumulation in the livers of the Atp7b-/(Wilson disease gene) knockout mice. Am J Pathol 168(2):423–434
Stabin MG (1996) MIRDOSE: personal computer software for internal dose assessment in nuclear medicine. J Nucl Med 37:538–546
Stabin MB, Siegel JA (2003) Physical models and dose factors for use in internal dose assessment. Health Phys 85:294–310
Hays MT, Watson EE, Thomas SR, Stabin M (2002) Radiation absorbed dose estimates from 18F-FDG. J Nucl Med 43:210–214
Blower PJ, Lewis JS, Zweit J (1996) Copper radionuclides and radiopharmaceuticals in nuclear medicine. Nucl Med Biol 23:957–980
Chelly J, Tumer Z, Tonnesen T, Petterson A, Ishikawa-Brush Y, Tommerup N, Horn N, Monaco AP (1993) Isolation of a candidate gene for Menkes disease that encodes a potential heavy metal binding protein. Nat Genet 3:14–19
Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J (1993) Isolation of a candidate gene for Menke’s disease and evidence that it encodes a copper-transporting. Nat Genet 3:7–13
Mercer JF, Livingston J, Hall B, Paynter JA, Begy C, Chandrasekharappa S, Lockhart P, Grimes A, Bhave M, Siemieniak D (1993) Isolation of a partial candidate gene for Menke’s disease by positional cloning. Nat Genet 3:20–25
Prasad AS, Brewer GJ, Schoomaker EB, Rabbani P (1978) Hypocupremia induced by zinc therapy in adults. JAMA 240:2166–2168
Jayakumar S, Micallef-Eynaud PD, Lyon TD, Cramb R, Jilaihawi AN, Prakash D (2005) Acquired copper deficiency following prolonged jejunostomy feeds. Ann Clin Biochem 42(3):227–231
Spinazzi M, De Lazzari F, Tavolato B, Angelini C, Manara R, Armani M (2007) Myelo-optico-neuropathy in copper deficiency occurring after partial gastrectomy: do small bowel bacterial overgrowth syndrome and occult zinc ingestion tip the balance? J Neurol 254:1012–1017
Acknowledgments
The authors thank Anjali Gupta for technical support in PET-CT scanning. This project was funded partially by the Department of Radiology, University of Texas Southwestern Medical Center at Dallas, Texas, USA, and National Institutes of Health (R56DK084510 to SL). The production of Cu-64 at Washington University School of Medicine is supported by NCI grant R24 CA86307.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peng, F., Lutsenko, S., Sun, X. et al. Imaging Copper Metabolism Imbalance in Atp7b −/− Knockout Mouse Model of Wilson’s Disease with PET-CT and Orally Administered 64CuCl2 . Mol Imaging Biol 14, 600–607 (2012). https://doi.org/10.1007/s11307-011-0532-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-011-0532-0