Abstract
Bark beetles infest several pine tree species, often creating major economic losses. Biotic interactions between Scolytinae populations inhabiting Pinus sylvestris were analyzed using a new sampling method involving a two dimensional division of tree space resources into units and sections. The goal was to evaluate the effects of the type of available reproduction material on insect infestation. The P. sylvestris stands in this study included an analysis of bark beetles colonizing dead trees and uninfested living trees (trap trees). Analyzed trees were harvested and their stems divided into ten equal units; each unit was halved into two sections (half of the stem circumference). The colonization of dead trees by insects was evaluated immediately after cutting for infested trees and 2 months after cutting for trap trees. The type of breeding material significantly affected the species composition of bark beetles infesting P. sylvestris. The dead trees were colonized mostly by Trypodendron lineatum and Tomicus minor, and to a lesser extent by Tomicus piniperda, which dominated in trap trees. Tomicus piniperda and T. lineatum preferred thicker stems; however, T. minor, Hylurgops palliatus and Pityogenes bidentatus preferred thinner ones. The application of the new sampling method helped to increase the accuracy of niche segregation among insect species. The results of niche segregation indicate bark beetles exhibited spatial specialization in the use of resources, mainly related to the moisture content of breeding material and the availability of food resources which is the main factor determining the coexistence of bark beetles in the same environment.
Similar content being viewed by others
Introduction
When trees are weakened by various abiotic, biotic and anthropogenic factors, forest stands and cut trees become an important breeding base for bark beetles (Scolytinae; Dyer and Chapman 1965; Wood 1982; Jakuš 1998a, b; Sauvard 2004; Foit 2010). When beetles attack a tree this triggers defense responses, and the level of the response depends on the degree of tree weakening (Lieutier 2004). Bark beetles have developed specific mechanisms of infestation allowing the identification and selection of trees suitable for infestation, a phenomenon known in the literature as behavioral avoidance. Therefore, the physiological condition of trees has a significant effect on the species composition of tree-infesting cambio- and xylophagous insects (Borden 1997). The infestation of standing trees is closely related to the so-called threshold of successful attack (Christiansen et al. 1987) which may be exceeded as a result of: (1) decrease in tree resistance, (2) increase in population density size, or (3) when both of these situations occur simultaneously. Because cut trees lack resistance to beetle attack, they are often used as natural traps for predicting and reducing the populations of bark beetles such as the common pine shoot beetle Tomicus piniperda (L.) that commonly attacks Pinus sylvestris (L). In Europe, traps used for monitoring the population size of this species are made of whole trees or tree trunks (Grégoire and Evans 2004). In Poland, trap trees are usually placed in forest stands that have already been damaged by primary pests or pathogenic fungi and in the forest stands located near sawmill timber yards (Szujecki 1987). The stored unbarked wood of P. sylvestris is a breeding site of T. piniperda and the lesser pine shoot beetle Tomicus minor Hart. After emerging, the beetles of both species migrate to adjacent forests, where they feed on the shoots of healthy pines causing disturbances in crown development and, consequently, significant losses in volume increment (Ericsson et al. 1985; Långström and Hellqvist 1990, 1991; Långström et al. 1990; Czokajlo et al. 1997; Borkowski 2001).
The presence of more than one species of bark beetle attacking a single weakened tree may have positive and negative effects on beetle populations such as a combined effect that helps the beetles to overcome tree resistance or to increase competitive interactions among the beetle species (Light et al. 1983; Wagner et al. 1985; Flamm et al. 1987; Rankin and Borden 1991; Schlyter and Anderbrant 1993). Although many studies have addressed the effects of biotic interactions on the populations of species from different taxonomic groups (e.g. Mysterud 2000; Julliard et al. 2006; Dolédec et al. 2008; Young 2008), only a few publications have been conducted related to bark beetles. A few studies describe the biotic interactions of insects from the subfamily Scolytinae in spruce Picea abies L. (Karst.) (Grünwald 1986) or in pine species such as P. sylvestris (Amezaga and Rodríguez 1998; Borkowski 2013), Pinus radiata (D. Don) (Amezaga and Rodríguez 1998), Pinus resinosa Ait. (Ayres et al. 2001) and Pinus taeda (L.) (Paine et al. 1981). This type of research is very labor-intensive. It requires debarking of entire trees and precisely counting egg galleries. However, knowledge related to the distribution and density of egg galleries in trunks is essential for the description of biocoenotic interactions between bark beetles and host plants as well as the related mechanisms explaining the coexistence of various bark beetle species in the same habitat. In practice, the selection of a sampling method should allow the separation of the niches of all bark beetle species in accordance with Gause’s principle (Krebs 2009). This rule states that two species with similar environmental requirements can coexist only when they use available resources in different ways. The conditions of such coexistence can be accurately described by applying a method employing a factor (niche breadth, sampling method) that allows precise niche segregation for all the examined bark beetle species. This method could help researchers determine the nature of biotic interactions between bark beetles, which can be used in the protection of forest against pest insects.
The methods adopted in earlier studies using bark thickness as a factor related to niche segregation in bark beetles were not fully effective (Grünwald 1986; Amezaga and Rodríguez 1998). This occurred because various parts of individual trees may have different levels of suitability as food for bark beetles depending on the system and intensity of the entire complex of environmental factors affecting food resources (phloem, cambium, wood). Hence, the aim of the present study, carried out in 2009–2010, was to develop a new sampling method based on the assumption that the partitioning of spatial resources of trees into two dimensions more accurately reflects the usefulness of different parts of trees as this relates to their colonization by bark beetles. In addition, the effect of the type of the breeding material on the colonization of P. sylvestris by bark beetles was evaluated.
Materials and methods
Fieldwork
Observations were conducted in P. sylvestris forest stands in central Poland (50°55′N, 20°45′E; 350 m a.s.l.) infested by T. piniperda. Previous studies using marked beetles showed that the dispersion of the population of the greater pine shoot beetle from stored unbarked pine wood to adjacent forest stands can be within ca 2000 m from a sawmill yard (Barak et al. 2000; Poland et al. 2000). Therefore, the present study used observations involving stands with well-developed crowns, growing outside the area of mass attacks of pine-shoot beetles, that is, more than 3 km away from sawmill yards. In addition, all selected stands were located in upland mixed deciduous forest habitat. Scots pine (approximately 80 years old) and European beech (Fagus sylvatica L.; 65–90 years old) comprised about 40 % of the species composition of the stands; silver fir (Abies alba Mill.; 80–85 years old) and oaks (Quercus spp.; 75 years old) each comprised about 10 % of the forest stands.
In April 2009, 30 standing dead Scots pine trees were randomly selected in which the northern (N) and southern (S) parts in the butt end of the trunks were marked (Fig. 1a). After cutting, tree branches were removed, and both parts of the trunks (N and S) were permanently marked along their entire length of which each was half of the tree circumference (Fig. 1a). These are hereafter referred to as the “dead trees.” In February 2009 and 2010, 10 trap trees were prepared each year (a total of 20 trap trees) from the cut pines which were delimbed and placed on supports at a height enabling colonization of trunks by insects on their entire circumference (Fig. 1b). The traps trees consisted of physiologically healthy trees with a diameter and height similar to those of the dead trees; these trees were well-developed along their entire length (without technical defects). The parameters of environmental factors were assumed to change along a gradient from the base to the top and on the circumference of the trees. Therefore, a new method for distribution of sampling sites was developed as being more representative in characterizing the effects of environmental variables on the quality of food resources used by bark beetles. The upper (a) and lower (b) parts of the trunks of trap trees were permanently marked along their entire length, each part being one half of the circumference (Fig. 1b). Then, all the trunks of the dead and trap trees were divided into ten equal units (h), with two sections each, where:
a Standing dead trees (i) and trees after cutting and branch removal (ii); letters N and S indicate the northern and southern sections of dead trees, respectively. b Healthy pine (i) used as a trap tree (ii); letters U and L indicate the upper and lower section of the trap tree, respectively. Numbers 1, 2 ,…, 10 indicate stem units
-
h 1, h 3, …, h 19 is the odd index number of indicated sections comprising the upper part (trap trees) or the northern part (dead trees) of the next stem;
-
h 2, h 4, …, h 20 is the even index number of indicated sections comprising the lower part (trap trees) or southern part (dead trees) of the next stem.
Measurements were carried out for each tree, including:
-
stem diameter at the thickest part (butt end) and the thinnest part (top);
-
diameter of each of the ten stem units measured at the thicker end;
-
the total length of a fallen tree.
The mean diameter of dead trees was 30.8 cm (±5.3 SD) and was similar than the diameter of trap trees (28.8 cm ± 5.1 SD).
Tree infestation parameters were estimated using the method of entomological section-based analysis performed in dead trees immediately after cutting, and in trap trees cut in May of each year, when the mean length of T. piniperda egg galleries ranged from 8 to 10 cm.
The infestation of individual stem sections was determined by counting (1) the number of entrance holes Trypodendron lineatum Ol., (2) the number of maternal galleries T. piniperda, and (3) the number of egg galleries of T. minor, Pityogenes bidentatus Herbst. and Hulurgops palliatus Gyll.
The stem form of a coniferous tree can be expressed by Kunze’s equation (Eq. 1; Inoue 2006):
where r is stem radius, l is stem length from tree tip, b and c are coefficients. The stem surface area s of the tree can be computed by Eq. (2):
where h is the length of the fallen tree without top.
The total colonization density of each P. sylvestris stem was calculated: (1) after summing of bark beetles egg galleries in all sections and (2) after calculating the stem surface area.
Assessment of biotic interactions in the populations of bark beetle species
The niche breadth of species i was calculated using the Levin’s method (1968) based on Eq. (3):
where p ih is the proportion of a species i in section h; h is the stem section being half the circumference of the tree unit and n is the number of sections.
The niche breadth index reaches 20 if the use of the resources by a species is uniform along the entire length of the trap trees.
The assessment of the interactions among bark beetle species was based on the niche overlap index α ij calculated with the Levin’s method (1968), based on Eq. (4):
where p ih and p jh are the proportions of species i and j in section h; h is the stem section being half the circumference of the tree unit; n is the number of sections and B is the niche breadth of a species.
The α ij index reaches a value of 0 for two species that do not share the same resource of the environment.
The similarity between species distributions on a resource set was quantified using the estimate of proportional similarity (Feinsinger et al. 1981), based on Eq. (5):
where p ih and p jh are the proportions of species i and j in section h; h is the stem section being half the circumference of the tree unit and n is the number of sections.
It was assumed that niche segregation occurred when the value of the coefficient was less than 0.7 (Hutchinson 1959; Topp et al. 1982).
Statistical analysis
The assessment of biotic interactions among the individuals in the bark beetle populations was based on the equations used in similar studies related to the ecology of bark beetles (Paine et al. 1981; Grünwald 1986; Schlyter and Anderbrant 1993; Amezaga and Rodríguez 1998).
The data were analyzed for treatment effects using a one-way analysis of variance (ANOVA). Normality and homogeneity of the variances were checked using the Shapiro–Wilk and Levene tests, respectively, prior to ANOVA (Sokal and Rohlf 2012). The differences in the niche breadth of bark beetles in trap trees were compared using Tukey’s least significant difference test. The data were subjected to logarithmic transformation prior to statistical analysis. The differences in the niche breadth of T. lineatum and T. minor in dead trees were tested using a t test for independent variables. The homogeneity of variance for the data concerning niche overlap of bark beetles as a result of the applied transformations was not obtained. Therefore, trap trees were tested with the parametric Kruskal–Wallis test, and dead trees were tested using the Mann–Whitney U test. The t test for a single sample was used to assess the difference between the theoretical and empirical values of the coefficient of proportional similarity. These analyses were analyzed using the STATISTICA statistical software package v 10.0, StatSoft Inc. (StatSoft Inc 2010).
Results
Analysis of tree infestation by bark beetles
Stem diameters of trap trees were normally distributed (Shapiro–Wilk test: W = 0.9311; P = 0.1549; N = 20); however, for dead trees it was close to the normal distribution (Shapiro–Wilk test: W = 0.8349; P = 0.0351; N = 30), which indicates that the analysis involved tree samples with the dimensions close to the means.
The main Scolytinae species found in the dead trees were T. lineatum and T. minor which infested 100 and 80 % of pines, respectively. Egg galleries of T. piniperda were found sporadically in four trees with the mean density of 0.71 ± 2.57 SD maternal gallery/m2. The mean infestation density of T. lineatum in pine stems amounted to 97.1 ± 75.3 SD entrance holes/m2, and was nearly 2-fold higher than the mean infestation density of T. minor (53.5 ± 44.5 SD egg galleries/m2).
The beetle assemblages found in the examined trap trees consisted of four species: T. piniperda (100 % colonization of trees), T. minor (90 %), H. palliatus (90 %), P. bidentatus (65 %). Assemblages consisting, respectively, of two, three and four insect species were found in the same trees. The mean density of T. piniperda colonizing the trap trees, expressed as the number of maternal galleries/m2 was 51.7 ± 18.9 SD was nearly 5-fold higher than the mean density of T. minor (10.8 ± 9.9 SD) and 25-fold higher than the mean density of P. bidentaus (2.2 ± 3.5 SD) and H. palliatus (2.1 ± 2.8 SD).
The infestation density indices for both the dead and trap trees varied widely. The coefficients of variation for T. lineatum and T. minor in the dead trees accounted for 78 and 83 % of the total variation, respectively; however, for the trap trees they were 37 % for T. piniperda, 92 % for T. minor, 133 % for H. palliatus and 159 % for P. bidentatus, respectively.
Trypodendron linetaum colonized dead trees in the first six units of the stem counting from the butt end (Fig. 2a). The highest stem infestation density was noted in unit No. 2 (356.7 ± 295.9 SD entrance holes/m2), and the smallest in unit No. 6 (9.1 ± 27.7 SD entrance holes/m2). The population density of T. lineatum in the analyzed dead trees was found to decrease with the distance from the thickest part of the stem. Similarly, T. piniperda colonized the trap trees along the entire length of the stems mostly in their thicker part (Fig. 3a). The spatial distribution of egg galleries was characterized by a slow decrease in the density of this species along the entire length of the stems. The greatest infestation was noted for unit No. 1, and the lowest for unit No. 10. The mean infestation density was 109.7 ± 55.7 SD and 1.8 ± 3.5 SD maternal galleries/m2, respectively.
Tomicus minor infested the dead and trap trees along their entire length, regardless of the size of its population, except for the first, thickest section (Figs. 2b, 3b). The infestation density of the analyzed dead and trap trees by lesser pine shoot beetles decreased with the distance from the most infested section. The decline was more pronounced for the trap trees characterized by a higher density of egg galleries in the thinner part of the trunk (Fig. 3b). The highest infestation was found in unit No. 9, and the smallest in unit No. 2. The mean infestation densities were 202.1 ± 191.3 SD and 2.6 ± 6.9 SD egg galleries/m2 in the dead trees and 23.7 ± 40.9 SD and 0.7 ± 1.0 SD egg gallery/m2 in the trap trees.
The infestation pattern of trap trees by P. bidentatus and H. palliatus was similar to the infestation pattern of trap trees by T. minor, except for the infestation culmination zone (Fig. 3c, d). The largest infestation occurred in unit No. 7 amounting to 12.7 ± 18.7 SD egg galleries/m2 for P. bidentatus and 9.7 ± 19.4 SD egg galleries/m2 for H. palliatus.
The analysis of the distribution of egg galleries in the upper and lower sections of the trap trees and in the northern and southern sections of the dead trees showed no significant differences in stem colonization by bark beetles with only exception. That is, H. palliatus colonized only the lower part of the trap trees. The percentage share in the colonization of the upper and lower sections of the trap trees was 48–52 % for T. piniperda, 53–47 % for T. minor, 55–45 % for P bidentatus, and in the case of the northern and southern sections of the dead trees it was 55–45 % for T. lineatum and 49–51 % for T. minor.
Analysis of biotic interactions in the populations of bark beetle species
Niche breadth
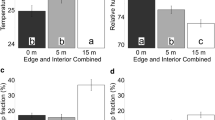
The range of resources of dead pine trees exploited by T. minor was significantly larger than by T. linetaum (t = 7.2659; df = 52; P < 0.001; Fig. 4a). The mean niche breadth of T. minor was 9.1 ± 1.9 SD and was almost 2-fold larger than the mean niche breadth of T. lineatum (5.2 ± 1.9 SD).
Niche breadth (means and standard errors) of the bark beetle species colonizing dead trees a Tomicus minor, Trypodendron lineatum and trap trees b Tomicus piniperda, T. minor, Pityogenes bidentatus, Hylurgops palliatus: a, b means marked with different letters indicate statistically significant differences among the bark beetle species in dead (t test P < 0.05) and trap trees (Least significant difference (LSD) per Tukey’s LSD test, P < 0.05)
In the trap trees, the niche breadth indices for the populations of T. piniperda and T. minor were similar and significantly higher than those calculated for the populations of P. bidentatus and H. palliatus (F = 53.4142; df = 65; P < 0.001; Fig. 4b). The mean niche breadths for T. piniperda (B = 10.6 ± 2.0 SD) and T. minor (B = 9.1 ± 2.5 SD) were approximately 2 and 3-fold higher than the niche breadths for P. bidentatus (B = 5.3 ± 2.2 SD) and H. palliatus (B = 3.3 ± 0.7 SD), respectively.
Niche overlap
Pine-colonizing bark beetle species exhibited a level of coexistence in the trap trees than in the dead trees. No significant differences in the dominance of any of the species in exploiting the same resources was found during the analysis of the degree of niche overlap in dead trees (Mann–Whitney’s U test: Z = 0.5463; P = 0.5848; Fig. 5a). For greater pine shoot beetles the index values of the degree of niche overlap in the trap trees were significantly lower for the species pairs sharing food resources (Kruskal–Wallis ANOVA: H df=11 (N = 186) = 122.6862; P < 0.001; Fig. 5b). The use of the same resources as H. palliatus by Pityogenes bidentatus and T. minor revealed the strongest coexistence between the analyzed pairs of bark beetle species. This was expressed in the value of the niche overlap index which was significantly higher than its value for the pairs of species with T. piniperda (Fig. 5b). The niche overlap index values for T. minor and P. bidentatus, as well as that for H. palliatus and P. bidentatus, were significantly higher than the values of this index for T. minor, P. bidentatus and H. palliatus sharing resources with T. piniperda. In other combinations of the pairs of bark beetle species, the exploitation of the same resources was on a similar level. No significant dominance of one particular species within the individual pairs of bark beetle species was found in the separation of the shared food resources of the trap trees, expressed in the niche overlap index.
Niche overlaps of the bark beetle species colonizing dead trees (a) and trap trees (b): a, b, c, d the value of niche overlaps marked with different letters indicate statistically significant differences among the bark beetle species in dead (t test, P < 0.05) and trap trees (Tukey’s Least significant difference test, P < 0.05). Pairs of individual bark beetle species were described by the following symbols (Hp Hylurgops palliatus, Pb Pityogenes bidentatus, Tm Tomicus minor, Tp Tomicus piniperda, Tl Trypodendron lineatum)
The niche of T. minor was found to have occupied a larger portion of the niche of H. palliatus (Fig. 5b). Only the niche of T. minor was found to occupy a larger portion of the niche of H. palliates (Fig. 5b).
Niche segregation
The niches of bark beetle species in both trap and dead trees were segregated. This was reflected in the significantly lower values of the coefficient of proportional similarity compared to the theoretical value of 0.7 (Table 1). T. lineatum and T. minor colonizing the dead trees showed the strongest spatial specialization (C = 0.03). In the trap trees, the higher values of the coefficient of proportional similarity, and thus the stronger coexistence, were found for H. palliatus, T. minor and P. bidentatus colonizing stems in their thinner parts.
Discussion
Tree infestation
The high rates of infestation of trees by T. lineatum in the study area strongly weakened the trees in the stand (Byers 2004). This is also confirmed by the low percentage share of T. piniperda in the species composition (it infested four trees) which, in the succession of the insects developing in dead pines, typically infests trees prior to T. minor (Bakke 1968; Långström and Hellqvist 1985). The infestation rate of both the dead and trap trees varied, as evidenced by the very high values of the coefficients of variation ranging from 37 % for T. piniperda to 159 % for P. bidentatus. Differences in the infestation rate of trees have been observed in many groups of forest insects, especially for bark beetles (e.g. Fargo et al. 1978; Bouhot et al. 1988; Chen and Tang 2007). In comparison with other bark beetle species, the relatively low value of the coefficient of variation of tree colonization by T. piniperda may, in addition to the various trophic values of trunks, be caused by the tree selection mechanism for colonization.
It is generally believed that the primary attraction phase plays a significant role in the colonization of trees by T. piniperda. This phase consists of the beetles locating dead trees over a great distance through the emission of specific volatile compounds by the trees (mainly α-pinene); however, the role of the pheromone information system is less important (Långström and Hellqvist 1985; Niemeyer et al. 1996; Poland et al. 2003). Attracted by a strong signal from all of the examined trap trees, the beetles colonized them uniformly on the entire surface area. Given the smaller role of the pheromone information system in tree colonization by T. piniperda, stridulation (mechanical information) of individuals becomes a possible mechanism for preventing the excessive population density in a given part of the stem (Rudinsky and Ryker 1976; Nilssen 1978). The low colonization rate for the stems by T. lineatum in their thickest part may be caused by a mechanical barrier; that is, the thickest bark may prevent beetles from chewing into the phloem. This phenomenon was observed in, inter alia, T. piniperda for which bark thickness exceeding 31 mm was too great of a barrier (Saarenmaa 1983).
Biotic interactions
The sampling method used here, based on the division of trees into equal parts along the longitudinal and transverse axis of the stem, assumed that the factor responsible for the distribution of bark beetle species has an even effect on the entire length and circumference of the trees. One can assume that the parameters of the environmental factors change along the gradient from the base to the top of the stem and on the circumference of the trees. Based on that assumption, the method presented here of a uniform distribution of sampling sites is more representative in characterizing the effect of environmental variables on the quality of food resources used by bark beetles. This method allows an analysis based on one, though unspecified, factor for conditioning a stem prior to infestation. In previous studies, the applied segregation factors such as bark thickness or linear division of tree stems into equal parts for the selected pairs of bark beetle species were ineffective. No niche segregation was found for I. sexdentatus and T. piniperda in the stands of younger age classes in admixture with P. radiata and P. sylvestris (Amezaga and Rodríguez 1998) and for H. palliatus and P. bidentatus in the pine stands of older age classes (Borkowski 2013). As concerns P. abies, no niche segregation was observed for the following pairs of bark beetle species: Crypturgus pusillus (Gyll.) and Ips typographus (L.), I. typographus and Orthotomicus suturalis (Gyll.) as well as C. pusillus and O. suturalis (Grünwald 1986). The original sampling method presented here based on a uniform partitioning of food resources is fully effective. This is confirmed by the presence of niche segregation for all bark beetles species (Table 1). The examination of the trap trees proves this method is very efficient even with the significant niche overlap (Fig. 5b). Note that in the case of a larger number of species with similar ecological requirements, it is possible to increase the accuracy of the segregation factor, involving the division of tree circumference into, for example, four equal parts (top, two lateral and base).
The comparison of the degree of niche overlap in the trap and dead trees indicates that the intensity of interspecific interactions depends primarily on the availability of the resources suitable for infestation. These resources are directly proportional to the lateral surface area of a tree and inversely proportional to the population density. Although the examined pines had similar diameters (see the “Materials and methods” section), the dead tree resources were more restricted (very high population density of T. lineatum and T. minor) than those of the trap trees (very high density of T. piniperda and very low density of the remaining species in the assemblage). The very low degree of niche overlap observed in both bark beetle species in the dead trees may indicate a strong competition in resource use among the individuals of both populations in the case of restricted resources (Levins 1968; Krebs 2009). As a result, T. lineatum and T. minor colonized the thicker and thinner part of the trees, respectively. However, a comparison of niche overlap indices of the examined pines shows a much stronger separation of resources in the dead trees (complete niche separation occurs at about half of the trees, as evidenced by the position of the median) than might be expected in a situation under the conditions where the two species do not compete directly for the same resources (space dimensions). The comparison of the biology of both species shows that T. minor colonizes the wood surface exclusively, as opposed to T. lineatum whose life cycle takes place inside the wood. The cause of a very poor coexistence of the two Scolytinae species infesting dead trees can be sought in the preference of T. lineatum for a moister breeding material since it is an ambrosia beetle species depending on its own symbiotic fungus. This is supported by the presence of this species only in the thick part of the stem and its absence in the trap trees which, placed on the supports in locations temporarily exposed to the sun, lost moisture faster, making them less suitable for colonization by this species. This is also confirmed by studies conducted under similar environmental conditions demonstrating that T. lineatum attacks mainly wood stored in the shade and not placed on supports (Dominik and Starzyk 2004) as well as typically attacks fresh wood immediately after being cut (Szmidt and Luterek 1999). A similar niche breadth of T. minor in the standing and trap trees, and a significantly larger area occupied by this species in the dead trees compared to T. lineatum is indicative of a greater range of tolerance revealed by T. minor in regard to the moisture content of the breeding material.
The relatively high degree of niche overlap in the trap trees was evidenced by a significantly larger share of the niches of P. bidentatus and T. minor in the niche of H. palliates. This overlap could be linked, in part, to the greater availability of food resources resulting from the low population density of the species present in the thinner part of the trees. It could also be related to the ecological specialization regarding the moisture content of the breeding material of the two smallest bark beetle species. In all the trap trees, egg galleries of H. palliatus were found to occur only in the lower sections, which is consistent with the ecological requirements of this species. H. palliatus is a hygrophilous organism, principally occurring in shaded and moist environments, and colonizing stems along their entire length excluding branches. In contrast, P. bidentatus colonizes stems along the entire circumference including branches (Szujecki 1987). In addition, other researchers (Glasser and Price 1988) observed an increase in niche overlap in when resources were nearly unlimited resources for, inter alia, T. piniperda (Amezaga and Rodríguez 1998). This may indicate a low degree of competitive interaction for living space among individuals of the coexisting species (Colvell and Futuyma 1971; Ratchke 1976; Lawlor 1980).
The model developed by Price (1997) used for the description of tree infestation patterns shows a positive correlation between the body size of a beetle with the thickness of the phloem and area of the exploited resources. The presented relationships partly confirm the results of the studies carried out in the trap trees. Pityogenes bidentatus and H. palliatus, the species having the smallest body size, mainly colonize the thinner part of the stem, and their niche breadths are significantly smaller than are the niche breadths of both pine shoot beetles species (Fig. 2). However, the model developed here is ineffective for the greater bark beetles species. The thickness of the phloem decreases with the increasing distance from the base toward the top of the trunk. Therefore, if phloem thickness is the most important variable for describing the infestation patterns of species with different body sizes, then T. piniperda should not colonize trees with the thinnest bark layer (Fig. 2a). Amezaga and Rodríguez (1998) pointed out similar limitations of the model for I. sexdentatus and T. piniperda, two species that colonized parts of the trap trees with bark thickness 0–2 mm, and by Paine et al. (1981) for four species of different body size colonizing P. taeda in North America.
The results confirmed the well-known relationship that the type of breeding material available has a significant effect on the species composition and abundance of the major populations of bark beetles infesting P. sylvestris (Långström 1984; Sauvard 2004). A diverse range of tolerance for each species was found to be the main factor determining the level of coexistence of bark beetles in the same habitat. This range of tolerance is mainly related to the moisture content of the breeding material and the availability of food resources.
In the assessment of biotic interactions, the application of the new method of sample collection from trees that involves dividing the tree circumference into two sections helped to increase the accuracy of niche segregation of all bark beetles species under discussion. In the case of the co-existence of more species with similar ecological requirements, the applied sampling method allows a precise analysis of the niches occupied by various species of bark beetles. In turn, these data may help researchers analyze the interactions between the species of bark beetles colonizing the cut or downed trees of P. sylvestris and can be used to improve forest health through the monitoring of insect populations (Grégoire and Evans 2004).
References
Amezaga I, Rodríguez MA (1998) Resource partitioning of four sympatric bark beetles depending on swarming dates and tree species. For Ecol Manag 109:127–135
Ayres BD, Ayres MP, Abrahamson MD, Teale SA (2001) Resource partitioning and overlap in three sympatric species of Ips bark beetles (Coleoptera: Scolytidae). Oecologia 128:443–453
Bakke A (1968) Ecological studies on bark beetles (Coleoptera: Scolytidae) associated with Scots pine (Pinus sylvestris L.) in Norway with particular reference to the influence of temperature. Med Norsk Skog 21:441–602
Barak AV, McGrevy D, Tokaya G (2000) Dispersal and re-capture of marked, overwintering Tomicus piniperda (L.) (Coleoptera: Scolytidae) from Scotch pine bolts. Great Lake Entomol 33:69–80
Borden JH (1997) Disruption of semiochemical-mediated aggregation in bark beetles. In: Cardé RT, Minks AK (eds) Insect pheromone research: new directions. Chapman and Hall, New York, pp 421–438
Borkowski A (2001) Threats to pine stands by the pine shoot beetles Tomicus piniperda (L.) and T. minor (Hart.) around a sawmill in southern Poland. J Appl Entomol 125:1–4. doi:10.1046/j.1439-0418.2001.00580
Borkowski A (2013) The use of trap trees by larger pine-shoot beetle Tomicus piniperda (L.). Ecology and modelling. Jan Kochanowski University Press, Kielce, p 85 (In Polish)
Bouhot L, Lieutier F, Debouzie D (1988) Spatial and temporal distribution of attacks by Tomicus piniperda L. and Ips sexdentatus Boern. (Col., Scolytidae) on Pinus sylvestris. J Appl Entomol 106:356–371. doi:10.1111/j.1439-0418.1988.tb00604
Byers JA (2004) Chemical ecology of bark beetles in a complex olfactory landscape. In: Lieutier F, Day KR, Battisti A, Grégoire JC, Evans HF (eds) Bark and wood boring insects in living trees in Europe, a synthesis. Kluwer Academic Publishers, Dordrecht/Boston/London, pp 89–134
Chen H, Tang M (2007) Spatial and temporal dynamics of bark beetles in Chinese white pine in Qinling Mountains of Shaanxi Province, China. Environ Entomol 36:1124–1130
Christiansen E, Waring RH, Berryman AA (1987) Resistance of conifers to bark beetle attacks: searching for general relationships. For Ecol Manag 22:89–106. doi:10.1016/0378-1127(87)90098-3
Colvell RK, Futuyma DJ (1971) On the measurements of niche breadth and overlap. Ecology 52:567–576. doi:10.2307/1934144
Czokajlo D, Wink RA, Warren JC, Teale SA (1997) Growth reduction of Scots pine, Pinus sylvestris, caused by the larger pine shoot beetle, Tomicus piniperda (Coleoptera, Scolytidae), in New York State. Can J For Res 27:1394–1397
Dolédec S, Chessel D, Gimaret-Carpentier C (2008) Niche separation in community analysis: a new method. Ecology 81:2914–2927
Dominik J, Starzyk JR (2004) Insects damaging a wood. PWRiL, Warsaw (In Polish)
Dyer ED, Chapman JA (1965) Flight and attack of the ambrosia beetle, Trypodendron lineatum (Oliv.) in relation to felling date of logs. Can Entomol 97:42–57
Ericsson A, Hellqvist C, Långström B, Larsson S, Tenow O (1985) Effects on growth of simulated and induced shoot pruning by Tomicus piniperda as related to carbohydrate and nitrogen dynamics in Scots pine (Pinus sylvestris). J Appl Ecol 22:105–124
Fargo WS, Coulson RN, Pulley PE, Pope DN, Kelley CL (1978) Spatial and temporal patterns of within-tree colonization by Dendroctonus frontalis (Coleoptera: Scolytidae). Can Entomol 110:1213–1232
Feinsinger PE, Spears E, Poole RW (1981) A simple measure of niche breadth. Ecology 62:27–32
Flamm RO, Wagner TL, Cook SP, Pulley PE, Coulson RN, McArdle TM (1987) Host colonization by cohabiting Dendroctonus frontalis, Ips avulsus, and I. calligraphus (Coleoptera: Scolytidae). Environ Entomol 16:390–399
Foit J (2010) Distribution of early-arriving saproxylic beetles on standing dead Scots pine trees. Agric For Entomol 12:133–141
Glasser JW, Price HJ (1988) Evaluating expectations deduced from explicit hypotheses about mechanisms of competition. Oikos 51:57–70
Grégoire JC, Evans HF (2004) Damage and control of BAWBILT organisms an overview. In: Lieutier F, Day KR, Battisti A, Grégoire JC, Evans HF (eds) Bark and wood boring insects in living trees in Europe, a synthesis. Kluwer Academic Publishers, Dordrecht/Boston/London, pp 19–39
Grünwald M (1986) Ecological segregation of bark beetles (Coleoptera, Scolytidae) of spruce. Z Angew Entomol 101:176–187
Hutchinson GE (1959) Homage to Santa Rosalia, or why are there so many kinds of animals? Am Nat 93:145–159
Inoue A (2006) A model for the relationship between form-factors for stem volume and those for stem surface area in coniferous species. J For Res 11:289–294. doi:10.1007/S10310-006-0210-2
Jakuš R (1998a) Patch level variation on bark beetle attack (Col., Scolytidae) on snapped and uprooted trees in Norway spruce primeval natural forest in endemic conditions: species distribution. J Appl Entomol 122:65–70
Jakuš R (1998b) Patch level variation on bark beetle attack (Col., Scolytidae) on snapped and uprooted trees in Norway spruce primeval natural forest in endemic conditions: effects of host and insolation. J Appl Entomol 122:409–421
Julliard R, Clavel J, Devictor V, Jiguet F, Couver D (2006) Spatial segregation of specialists and generalists in bird communities. Ecol Lett 9:1237–1244. doi:10.1111/j.1461-0248.2006.00977.x
Krebs CJ (2009) Ecology: the experimental analysis of distribution and abundance, 6th edn. Benjamin Cummings, San Francisco
Långström B (1984) Windthrown Scots pines as brood material for Tomicus piniperda and T. minor. Silv Fen 18:187–198
Långström B, Hellqvist C (1985) Pinus contorta as a potential host for Tomicus piniperda L. and T. minor (Hart.) (Col., Scolytidae) in Sweden. Zeitschr Angew Entomol 99:174–181. doi:10.1111/j.1439-0418.1985.tb01976.x
Långström B, Hellqvist C (1990) Spatial distribution of crown damage and growth losses caused by recurrent attacks of pine shoot beetles in pine stands surrounding a pulp mill in southern Sweden. J Appl Entomol 110:261–269. doi:10.1111/j.1439-0418.1990.tb00121.x
Långström B, Hellqvist C (1991) Shoot damage and growth losses following three years of Tomicus-attacks in Scots pine stand close to a timber storage site. Silva Fenn 25:133–145
Långström B, Tenow O, Eriksson A, Hellqvist C, Larsson S (1990) Effects of shoot pruning on stem growth, needle biomass, and dynamics of carbohydrates and nitrogen in Scots pine as related to season and tree age. Can J For Res 20:514–523
Lawlor LR (1980) Overlap, similarity and competition coefficients. Ecology 61(2):45–251
Levins R (1968) Evolution in changing environments: some theoretical explorations. Princeton University Press, Princeton
Lieutier F (2004) The BAWBILT context in Europe. In: Lieutier F, Day KR, Battisti A, Grégoire JC, Evans HF (eds) Bark and wood boring insects in living trees in Europe, a synthesis. Kluwer Academic Publishers, Dordrecht/Boston/London, pp 3–11
Light DM, Birch MC, Paine TD (1983) Laboratory study of intraspecific and interspecific competition within and between two sympatric bark beetle species, Ips pini and I. paraconfusus. Zeitschr Angew Entomol 96:233–241. doi:10.1111/j.1439-0418.1983.tb03664.x
Mysterud A (2000) The relationship between ecological segregation and sexual body size dimorphism in large herbivores. Oecologia 124:40–54. doi:10.1007/s004420050023
Niemeyer H, Watzek G, Lu N, Niu J (1996) Freilandtest zur Attraktivität von Monoterpenen und Ethanol für den Borkenkäfer Tomicus piniperda L. (Col., Scolytidae). J Appl Entomol 120:265–267. doi:10.1111/j.1439-0418.1996.tb01604.x
Nilssen AC (1978) Spatial attack pattern of the bark beetle Tomicus piniperda L. (Coleoptera: Scolytidae). Nor J Entomol 25:171–175
Paine TD, Birch MC, Švihra P (1981) Niche breadth and resource partitioning by four sympatric species of bark beetles (Coleoptera: Scolytidae). Oecologia 48:1–6
Poland TM, Haack RA, Petrice TR (2000) Dispersal of Tomicus piniperda (Coleoptera: Scolytidae) from operational and simulated mil yards. Can Entomol 132:853–866. doi:10.4039/Ent132853-6
Poland TM, Groot P, Burke S, Wakarchuk D, Haack RA, Nott R, Scarr T (2003) Development of an improved attractive lure for the pine shoot beetle, Tomicus piniperda (Coleoptera: Scolytidae). Agric For Entomol 5:293–300
Price PW (1997) Insect ecology, 3rd edn. Wiley, New York
Rankin LJ, Borden JH (1991) Competitive interactions between the mountain pine beetle and the pine engraver in lodgepole pine. Can J For Res 21:1029–1036. doi:10.1139/x91-141
Ratchke BJ (1976) Competition and coexistence within a guild of herbivorous insects. Ecology 57:76–87
Rudinsky JA, Ryker LC (1976) Sound production in Scolytidae: rivalry and premating stridulation of male Douglas-fir beetle. J Insect Physiol 22:997–1003
Saarenmaa H (1983) Modeling the spatial pattern and intraspecific competition in Tomicus piniperda (Coleoptera: Scolytidae). Commun Inst For Fenn 118:1–40
Sauvard D (2004) General biology of bark beetles. In: Lieutier F, Day KR, Battisti A, Grégoire JC, Evans HF (eds) Bark and wood boring insects in living trees in Europe, a synthesis. Kluwer Academic Publishers, Dordrecht/Boston/London, pp 63–89
Schlyter F, Anderbrant O (1993) Competition and niche separation between two bark beetles: existence and mechanisms. Oikos 68:437–447. doi:10.2307/3544911
Sokal RR, Rohlf FJ (2012) Biometry: the principles and practice of statistics in biological research. Freeman W. H. and Co., New York
StatSoft Inc (2010) Statistica for windows. StatSoft Inc Tulsa, Oklahoma
Szmidt A, Luterek R (1999) The impact of pine wood humidity on the colonisation Trypodendron lineatum Ol. Sylwan 143:77–81 (In Polish)
Szujecki A (1987) Ecology of forest insects. Springer, Netherlands
Topp W, Hansen K, Brandt R (1982) Artengemeinschaften von Kurzfuglern an Aas (Coleotera: Scolytidae). Entomol Gen 7:347–364
Wagner TL, Flamm RO, Coulson RN (1985) Strategies for cohabitation among the southern pine bark beetle species: comparisons of life-process biologies. South Fort Exp Stat 56:87–101
Wood SL (1982) The bark and ambrosia beetles of North and Central America (Coleoptera: Scolytidae), a taxonomic monograph. Great Basin Nat Mem 6:1–1359
Young KA (2008) Asymmetric competition, habitat selection, and niche overlap in juvenile salmonids. Ecology 85:134–149. doi:10.1890/02-0402
Acknowledgments
The authors would like to thank the workers of Forest District in Zagnansk for guidance and assistance in carrying out in this research. The Ministry of Science and Higher Education in Poland (Project no. 612464) funded this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Borkowski, A., Skrzecz, I. Ecological segregation of bark beetle (Coleoptera, Curculionidae, Scolytinae) infested Scots pine. Ecol Res 31, 135–144 (2016). https://doi.org/10.1007/s11284-015-1322-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-015-1322-y