Abstract
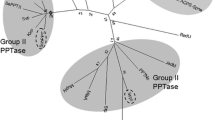
Malonyl-CoA:acyl-carrier protein transacylase (MCAT), which transfers the malonyl group from malonyl-CoA to holo-acyl carrier protein (ACP), is a key enzyme in fatty acid biosynthesis. Schizochytrium sp. TIO1101 is a marine protist with high levels of docosahexaenoic acid accumulation. In this study, the putative fabD gene coding MCAT was isolated from Schizochytrium sp. TIO1101. The Schizochytrium MCAT gene (ScTIOfabD) contained an 1176 bp open reading frame encoding a protein of 391 amino acids. The ScTIOfabD gene exhibited high novelty in nucleotide and amino acid sequence. The highest amino acid identity was only 35 % between ScTIOMCAT and the reported MCATs. Further studies demonstrated that ScTIOMCAT could bind malonyl-CoA directly and transfer malonyl group from malonyl-CoA to the ACP domain in vitro. Phylogenetic analysis suggested that ScTIOMCAT was relative close to MCATs of yeast strains. Overexpression of ScTIOMCAT in Saccharomyces cereviseae significantly increased the MCAT activity, without negative effects on the growth rate of the host strain. In addition, ScTIOMCAT generated 16.8 and 62 % increase in biomass and fatty acid accumulation, respectively, and did not alter the profile of fatty acid. Our results indicated that the novel MCAT gene from Schizochytrium sp. TIO1101 was crucial for fatty acid synthesis and had potential applications for genetic modifications of oil-producing species.




Similar content being viewed by others
References
Aguilar PS, de Mendoza D (2006) Control of fatty acid desaturation: a mechanism conserved from bacteria to humans. Mol Microbiol 62:1507–1514
Burja AM, Radianingtyas H, Windust A, Barrow CJ (2006) Isolation and characterization of polyunsaturated fatty acid producing Thraustochytrium species: screening of strains and optimization of omega-3 production. Appl Microbiol Biotechnol 72:1161–1169
Cavalier-Smith T, Allsopp M, Chao E (1994) Thraustochytrids are chromists, not fungi: 18 s rRNA signatures of heterokonta. Philos Trans R Soc Lond B Biol Sci 346:387–397
Cheng RB, Lin XZ, Wang ZK, Yang SJ, Rong H, Ma Y (2011a) Establishment of a transgene expression system for the marine microalga Schizochytrium by 18S rDNA-targeted homologous recombination. World J Microbiol Biotechnol 27:737–741
Cheng R, Ma R, Li K, Rong H, Lin X, Wang Z, Yang S, Ma Y (2011b) Agrobacterium tumefaciens mediated transformation of marine microalgae Schizochytrium. Microbiol Res 167:179–186
Cronan JE, Thomas J (2009) Bacterial fatty acid synthesis and its relationships with polyketide synthetic pathways. Methods Enzymol 459:395–433
Fan KW, Jiang Y, Faan YW, Chen F (2007) Lipid characterization of mangrove thraustochytrid-Schizochytrium mangrovei. J Agric Food Chem 55:2906–2910
Gong XG, Ji J, Xie J, Zhou Y, Zhang JY, Zhong WT (2006) Expression, purification, and bioactivity of GST-fused v-Src from a bacterial expression system. J Zhejiang Univ Sci B 7:13–19
Honda D, Yokochi T, Nakahara T, Erata M, Higashihara T (1998) Schizochytrium limacinum sp. nov., a new thraustochytrid from a mangrove area in the west Pacific Ocean. Mycol Res 102:439–448
Huang J, Jiang X, Zhang X, Chen W, Tian B, Shu Z, Hu S (2008) Expressed sequence tag analysis of marine fungus Schizochytrium producing docosahexaenoic acid. J Biotechnol 138:9–16
Jeon E, Lee S, Won JI, Han SO, Kim J, Lee J (2011) Development of Escherichia coli MG1655 strains to produce long chain fatty acids by engineering fatty acid synthesis (FAS) metabolism. Enzyme Microb Technol 49:44–51
Joshi VC, Wakil SJ (1971) Studies on the mechanism of fatty acid synthesis. XXVI. Purification and properties of malonyl-coenzyme A-acyl carrier protein transacylase of Escherichia coli. Arch Biochem Biophys 143:493–505
Keatinge-Clay AT, Shelat AA, Savage DF, Tsai SC, Miercke LJ, O’Connell JD 3rd, Khosla C, Stroud RM (2003) Catalysis, specificity, and ACP docking site of Streptomyces coelicolor malonyl-CoA:ACP transacylase. Structure 11:147–154
Liu W, Han C, Hu L, Chen K, Shen X, Jiang H (2006) Characterization and inhibitor discovery of one novel malonyl-CoA:acyl carrier protein transacylase (MCAT) from Helicobacter pylori. FEBS Lett 580:697–702
Magnuson K, Jackowski S, Rock CO, Cronan JE Jr (1993) Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol Rev 57:522–542
Metz JG, Roessler P, Facciotti D, Levering C, Dittrich F, Lassner M, Valentine R, Lardizabal K, Domergue F, Yamada A, Yazawa K, Knauf V, Browse J (2001) Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes. Science 293:290–293
Molnos J, Gardiner R, Dale GE, Lange R (2003) A continuous coupled enzyme assay for bacterial malonyl-CoA:acyl carrier protein transacylase (FabD). Anal Biochem 319:171–176
Neels JG, Olefsky JM (2006) Cell signaling. A new way to burn fat. Science 312:1756–1758
Ruch FE, Vagelos PR (1973) The isolation and general properties of Escherichia coli malonyl coenzyme A-acyl carrier protein transacylase. J Biol Chem 248:8086–8094
Serre L, Swenson L, Green R, Wei Y, Verwoert II, Verbree EC, Stuitje AR, Derewenda ZS (1994) Crystallization of the malonyl coenzyme A-acyl carrier protein transacylase from Escherichia coli. J Mol Biol 242:99–102
Summers RG, Ali A, Shen B, Wessel WA, Hutchinson CR (1995) Malonyl-coenzyme A: acyl carrier protein acyltransferase of Streptomyces glaucescens: a possible link between fatty acid and polyketide biosynthesis. Biochemistry 34:9389–9402
Sun M, Zhu G, Qin Z, Wu C, Lv M, Liao S, Qi N, Xie M, Cai J (2012) Functional characterizations of malonyl-CoA:acyl carrier protein transacylase (MCAT) in Eimeria tenella. Mol Biochem Parasitol 184:20–28
Verwoert II, van der Linden KH, Nijkamp HJ, Stuitje AR (1994) Developmental specific expression and organelle targeting of the Escherichia coli fabD gene, encoding malonyl coenzyme A-acyl carrier protein transacylase in transgenic rape and tobacco seeds. Plant Mol Biol 26:189–202
White SW, Zheng J, Zhang YM, Rock CO (2005) The structural biology of type II fatty acid biosynthesis. Annu Rev Biochem 74:791–831
Zhang Y, Cronan JE Jr (1998) Transcriptional analysis of essential genes of the Escherichia coli fatty acid biosynthesis gene cluster by functional replacement with the analogous Salmonella typhimurium gene cluster. J Bacteriol 180:3295–3303
Zhang X, Agrawal A, San KY (2011) Improving fatty acid production in Escherichia coli through the overexpression of malonyl coA-acyl carrier protein transacylase. Biotechnol Prog 28:60–65
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81202820), Zhejiang Provincial Natural Science Foundation of China (Grant No. LQ12H28005), Zhejiang Provincial Administration of Traditional Chinese Medicine (No. 2012ZQ006) and Scientific Research Fund of Zhejiang Provincial Education Department (No. Y201223804).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheng, R., Ge, Y., Yang, B. et al. Cloning and functional analysis of putative malonyl-CoA:acyl-carrier protein transacylase gene from the docosahexaenoic acid-producer Schizochytrium sp. TIO1101. World J Microbiol Biotechnol 29, 959–967 (2013). https://doi.org/10.1007/s11274-013-1253-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-013-1253-0