Abstract
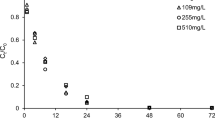
In this study, batch experiments have been carried out to investigate the mechanism of fluoride uptake by layered double hydroxide (LDH) and calcined layered double hydroxide (CLDH). Furthermore, practical use of these synthetic minerals was studied in continuous mini-column experiments. In these column studies, groundwater from Ethiopia was tested. LDH and CLDH were synthesized with Mg/Al mole ratio of 2. From batch experimental study, LDH and CLDH have shown maximum removal capacity of 84 and 222 mg F−/g from aqueous solution, respectively. It was observed that fluoride removal was pH dependent with favorable pH range of 5–7 (max. at pH 6). The mechanism of removal is suggested to be ion exchange for LDH and a memory effect followed by surface precipitation reaction for CLDH. The presence of other anions lowered defluoridation capacity of LDH in the order of PO4 3− > SO4 2− > NO3 − ≈ Cl−. From continuous experiments at 1 mM NaHCO3, LDH showed maximum defluoridation capacity of 1.3 mg/g and CLDH up to 20 mg/g. It was also observed that increase of bicarbonate concentration to 10 mM lowered the fluoride uptake capacity of CLDH to 4 mg/g. The presence of 1 mM H4SiO4 further reduced fluoride uptake capacity to 3 mg/g. CLDH column tested with groundwater from the Rift Valley with 10.5 mg F−/L has shown maximum removal capacity of 2.2 mg F−/g. Regeneration of this column indicated that CLDH has a good potential to be re-used.









Similar content being viewed by others
References
Arlappa, N., Aatif, Q. I., & Srinivas, R. (2013). Fluorosis in India: an overview. International Journal of Research and Development of Health, 1, 2321–1431.
Ashley, R. P., & Burley, M. J. (1994). Controls on the occurrence of fluoride in groundwater in the Rift Valley of Ethiopia. In H. Nash & G. J. H. McCall (Eds.), Groundwater quality (pp. 45–54). London: Chapman & Hall.
Ayenew, T. (2005). Major ions composition of the groundwater and surface water systems and their geological and geochemical controls in the Ethiopian Volcanic Terrain. Ethiopian Journal of Science, 28, 171–188.
Ayoob, S., & Gupta, A. K. (2006). Fluoride in drinking waters: a review on the status and stress effects. Critical Reviews in Environmental Science and Technology, 36, 433–487.
Bhatnagar, A., Kumar, E., & Sillanpaa, M. (2011). Fluoride removal from water by adsorption—a review. Chemical Engineering Journal, 171, 811–840.
Cavani, F., Trifiro, F., & Vaccari, A. (1991). Hydrotalcite-type anionic clays: preparation, properties and applications. Catalysis Today, 11, 173–301.
Dessalegne, M., & Zewge, F. (2013). Daily dietary fluoride intake in rural villages of the Ethiopian Rift Valley. Toxicological and Environmental Chemistry, 95, 1056–1068.
Diaz-Nava, C., Solache-Rios, M., & Olguin, M. T. (2003). Sorption of fluoride ions from aqueous solutions and well drinking water by thermally treated hydrotalcite. Separation Science and Technology, 38, 131–147.
Dissanayake, C. B. (1991). The fluoride problem in the groundwater of Sri Lanka environmental management and health. International Journal of Environmental Studies, 38, 137–155.
Duan, X., & David, G. E. (2006). Layered double hydroxides—structure and bonding. Berlin: Springer.
Eawag. (2015). Geogenic contamination handbook—addressing arsenic and fluoride in drinking water. Dubendorf: Switzerland: Swiss Federal Institute of Aquatic Science and Technology (Eawag).
Edmunds, M., & Smedley, P. (2005). Fluoride in natural waters. In O. Selinus, B. J. Alloway, J. A. Centeno, R. B. Finkelman, R. Fuge, U. Lindh, et al. (Eds.), Essentials of medical geology (pp. 301–330). London: Elsevier Academic Press.
Elhalil, A., Qourzal, S., Mahjoubi, F. Z., Elmoubarki, R., Farnane, M., Tounsadi, H., et al. (2016). Defluoridation of groundwater by calcined Mg/Al layered double hydroxide. Emerging Contaminants, 2(1), 42–48. doi:10.1016/j.emcon.2016.03.002.
EPA, U. S. (2008). National Secondary Drinking Water Regulations. In U. S. E. P. Agency (Ed.). Washington.
Gaines, G. L., & Thomas, H. C. (1953). Adsorption studies on clay minerals. II. A formulation of the thermodynamics of exchange adsorption. Journal of Chemical Physics, 21, 714–718.
Habuda-Stanic, M., Ravancic, M. E., & Flanagan, A. (2014). A review on adsorption of fluoride from aqueous solution. Materials, 7, 6317–6366.
Hussain, Z., Daosheng, L., Xi, L., & Jianxiong, K. (2015). Defluoridation by a Mg–Al–La triple-metal hydrous oxide: synthesis, sorption, characterization and emphasis on the neutral pH of treated water. RSC Advances, 5, 43906–43916.
Johnson, C. A., & Glasser, F. P. (2003). Hydrotalcite-like minerals (M2Al(OH)6 (CO3)0.5.XH2O, where M = Mg, Zn, Co, Ni) in the environment: synthesis, characterization and thermodynamic stability. Clays and Clay Minerals, 51, 1–8.
Kang, M. J., Chun, K. S., Rhee, S. W., & Do, Y. (1999). Comparison of sorption behavior of I- and TcO4- on Mg/Al layered double hydroxide. Radiochimica Acta, 85, 57–63.
Kummert, R., & Stumm, W. (1980). Surface complexation of organic acids on hydrous Al2O3. J Colloid Interface Sci, 75, 373–385.
Lazaridis, N. K., & Asouhidou, D. D. (2003). Kinetics of sorptive removal of chromium(VI) from aqueous solutions by calcined Mg–Al–CO3 hydrotalcite. Water Research, 37, 2875–2882.
Lv, L., He, J., Wei, M., & Duan, X. (2006a). Kinetic studies on fluoride removal by calcined layered double hydroxides. [Article]. Industrial and Engineering Chemistry Research, 45(25), 8623–8628. doi:10.1021/ie050363d.
Lv, L., He, J., Wei, M., Evans, D. G., & Duan, X. (2006b). Factors influencing the removal of fluoride from aqueous solution by calcined Mg-Al-CO3 layered double hydroxides. [Article]. Journal of Hazardous Materials, 133(1-3), 119–128. doi:10.1016/j.jhazmat.2005.10.012.
Lv, L., He, J., Wei, M., Evans, D. G., & Zhou, Z. (2007). Treatment of high fluoride concentration water by MgAl-CO3 layered double hydroxides: kinetic and equilibrium studies. [Article]. Water Research, 41(7), 1534–1542. doi:10.1016/j.watres.2006.12.033.
Miretzky, P., & Cirelli, A. F. (2011). Fluoride removal from water by chitosan derivatives and composites: a review. Journal of Fluorine Chemistry, 132, 231–240.
Miyata, S. (1983). Anion-exchange properties of hydrotalcite-like compounds. Clays and Clay Minerals, 31, 305–311.
Mulugeta, E., Zewge, F., Johnson, C. A., & Chandravanshi, B. S. (2014). A high-capacity aluminum hydroxide-based adsorbent for water defluoridation. Desalination and Water Treatment, 52, 28–30.
Oladoja, N. A., Drewes, J. E., & Helmreich, B. (2015). Assessment of fixed bed of aluminum infused diatomaceous earth as appropriate technology for groundwater defluoridation. Separation and Purification Technology, 153, 108–117.
Oladoja, N. A., & Helmreich, B. (2014). Batch defluoridation appraisal of aluminium oxide infused diatomaceous earth. Chemical Engineering Journal, 258, 51–56.
Oladoja, N. A., Liu, Y., Drewes, J. E., & Helmreich, B. (2016). Preparation and characterization of a reactive filter for groundwater defluoridation. Chemical Engineering Journal, 283, 1154–1167.
Orthman, J., Hu, H. Y., & Lu, G. Q. (2003). Use of anion clay hydrotalcite to remove coloured organics from aqueous solution. Separation and Purification Technology, 31, 53–59.
Palmer, S. J., Frost, R. L., & Nguyen, T. (2009). Hydrotalcites and their role in coordination of anions in Bayer liquors: anion binding in layered double hydroxides. Coordination Chemistry Reviews, 253, 250–267.
Paripurnanda, L., Saravanamuthu, V., Jaya, K., & Ravi, N. (2013). Defluoridation of drinking water using adsorption processes. Journal of Hazardous Materials, 248–249, 1–19.
Post, J. C., & Lundin, C. G. (1996). Guidelines for integrated coastal zone management. World Bank report.
Ruping, L., Xianggui, K., Zhao, N. Y., & Wencheng, L. G. (2013). Properties and application of layered double hydroides for electrochemical biosensors. Chemical Industry and Engineering Progree, 32, 2661–2667.
Sani, T., Adem, M., Fetter, G., Bosch, P., & Diaz, I. (2016) Defluoridation performance comparison of nano-hydrotalcite/hydroxyapatite composite with calcined hydrotalcite and hydroxyapatite. Water, Air, & Soil Pollution, 227(90), 1–8.
Steenbergen, F. V., Tekle-Haimanot, R., & Sidelil, A. (2011). High fluoride, modest fluorosis: investigation in drinking water supply in Halaba (SNNPR, Ethiopia). Journal of Water Resource and Protection, 3, 120–126.
Susheela, A. K., Bhatnager, M., Vig, K., & Mondal, N. K. (2005). Excess fluoride ingestion and thyroid hormone derangements in children living Delhi, India. Fluoride, 38, 151–161.
Tamiru, A. (1993). Preliminary analysis of the availability of groundwater in Ethiopia. Ethiopian Journal of Science, 16, 43–59.
Tekle-Haimanot, R. (2005). Study of fluoride and fluorosis in Ethiopia with recommendations on appropriate defluoridation technologies (Consultancy Report, pp. 1–55).
Tekle-Haimanot, R., Fekadu, A., & Bushura, B. (1987). Endemic fluorosis in the Ethiopian Rift Valley. Trop. Geogr. Med., 39, 209–217.
Tekle-Haimanot, R., Melkamu, Z., Kloos, H., Riemann, C., Fantaye, W., Zerihun, L., et al. (2006). The geographic distribution of fluoride in surface and ground water in Ethiopia with an emphasis on the Rift Valley. Science of The Total Environment, 367, 182–190.
Ulibarri, M. A., Pavlovic, I., Hermosin, M. C., & Cornejo, J. (1995). Hydrotalcite-like compounds as potential sorbents of phenols from water. Applied Clay Science, 10, 131–145.
WHO. (2011). Guidelines for drinking water quality (4th ed.). Geneva: WHO.
Wu, T., Mao, L., & Wang, H. (2015). Adsorption of fluoride on Mg/Fe layered double hydroxides material prepared via hydrothermal process. [Article]. RSC Advances, 5(30), 23246–23254. doi:10.1039/c4ra16839a.
Zeng, B., & Hong, Y. (1988). Geochemical environment related to human endemic fluorosis in China. In I. Thorton (Ed.), Geochemistry and Health (pp. 93–96). London: Science Reviews.
Zewge, F. (2005). Solution to the fluoride problem in the Rift Valley region of Ethiopia. Bulletin of the Chemical Society of Ethiopia, 14, 15–22.
Acknowledgments
The authors would like to dedicate this work to our colleague Annette Johnson for her contribution to Ethiopia. The authors would like to acknowledge the Swiss Federal Institute of Aquatic Science and Technology (Eawag) for the financial and technical support. MD acknowledges the Ethiopian Institute of Water Resources, Addis Ababa University. Special thanks to the staff of EAWAG and Prof. Gerhard Furrer from ETH-Zurich. ID is grateful to CSIC for her research leave at AAU and the Spanish Government, MINECO (MAT2012-31127), for funding.
Author information
Authors and Affiliations
Corresponding author
Additional information
C. Annette Johnson died before publication of this work was completed.
Rights and permissions
About this article
Cite this article
Dessalegne, M., Zewge, F., Pfenninger, N. et al. Layered Double Hydroxide and Its Calcined Product for Fluoride Removal from Groundwater of Ethiopian Rift Valley. Water Air Soil Pollut 227, 381 (2016). https://doi.org/10.1007/s11270-016-3079-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-3079-5