Abstract
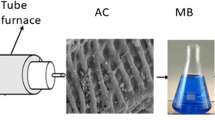
By using the activated carbon obtained from Citrullus lanatus rind by zinc chloride activation, methylene blue (MB) removal from aqueous solutions was studied, and the adsorption mechanism was solved through Weber-Morris intraparticle diffusion model, Bangham model, Boyd model, Fourier transform infrared spectra, and scanning electron microscopy. The effects of adsorption parameters (adsorbent concentration, temperature, initial dye concentration, and pH) were investigated. The equilibrium data of MB adsorption were described by applying the Langmuir, Freundlich, Temkin, and Dubinin-Radushkevich isotherm models. The obtained results from adsorption isotherms indicated that Langmuir model is the best-fitted model with the maximum adsorption capacities of 231.48, 243.90, 244.50, and 259.74 mg/g at 25, 35, 45, and 55 °C, respectively. The analysis of the kinetic data by pseudo-first-order, pseudo-second-order, and Elovich models displayed that MB adsorption followed pseudo-second-order model. Also, the date obtained from intraparticle diffusion model, Bangham model, and Boyd model presented that intraparticle diffusion, pore diffusion, and film diffusion played significant role in MB adsorption. The thermodynamic studies demonstrated that MB adsorption onto the activated carbon obtained from C. lanatus rind was physical, spontaneous, feasible, and endothermic. Thus, the activated carbon prepared from C. lanatus rind has been an efficient adsorbent for MB removal from an aqueous solution.

ᅟ









Similar content being viewed by others
References
Aharoni, C., & Ungarish, M. (1977). Kinetics of activated chemisorption. Part 2—theoretical models. Journal of the Chemical Society, Faraday Transactions, 73, 456–464.
Ai, L., Zhang, C., Liao, F., Wang, Y., Li, M., Meng, L., & Jiang, J. (2011). Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: kinetic, isotherm and mechanism analysis. Journal of Hazardous Materials, 198, 282–290.
Angın, D., Köse, T. E., & Selengil, U. (2013). Production and characterization of activated carbon prepared from safflower seed cake biochar and its ability to absorb reactive dyestuff. Applied Surface Science, 280, 705–710.
Boparai, H. K., Joseph, M., & O’Carroll, D. M. (2011). Kinetics and thermodynamics of cadmium ion removal by adsorption onto nanozerovalent iron particles. Journal of Hazardous Materials, 186, 458–465.
Boyd, G. E., Adamson, A. W., & Myers, L. S. (1947). The exchange adsorption of ions from aqueous solutions by organic zeolites. II. Kinetics. Journal of American Chemical Society, 69, 2836–2848.
Chien, S. H., & Clayton, W. R. (1980). Adsorption of hexavalent chromium from aqueous solutions by wheat bran. Soil Science Society of America Journal, 44, 265–268.
Deng, H., Lu, J., Li, G., Zhang, G., & Wang, X. (2011). Adsorption of methylene blue on adsorbent materials produced from cotton stalk. Chemical Engineering Journal, 172, 326–334.
Dubinin, M. M., & Radushkevich, L. V. (1947). The equation of the characteristic curve of activated charcoal. Proc Academy of Sciences of the USSR Physical Chemistry Section, 55, 331–337.
Ekrami, E., Dadashian, F., & Arami, M. (2015). Adsorption of methylene blue by waste cotton activated cotton: equilibrium, kinetics, and thermodynamic studies. Desalination and Water Treatment. doi:10.1080/19443994.2015.1015173.
Feng, Y., Yang, F., Wang, Y., Ma, L., Wu, Y., Kerry, P. G., & Yang, L. (2011). Basic dye adsorption onto an agro-based waste material—sesame hull (Sesamum indicum L.). Bioresource Technology, 102, 10280–10285.
Foo, K. Y., & Hameed, B. H. (2010). Insights into the modeling of adsorption isotherm systems. Chemical Engineering Journal, 156, 2–10.
Freundlich, H. M. F. (1906). Über die adsorption in lösungen. Zeitschrift fur PhysikalischeChemie, 57, 385–470.
Fu, J., Chen, Z., Wang, M., Liu, S., Zhang, J., Zhang, J., Han, R., & Xu, Q. (2015). Adsorption of methylene blue by a high efficiency adsorbent (polydopamine microspheres): kinetics, isotherm, thermodynamics and mechanism analysis. Chemical Engineering Journal, 259, 53–61.
Gao, Q., Liu, H., Cheng, C., Li, K., Zhang, C., & Li, Y. (2013). Preparation and characterization of activated carbon from wool waste and the comparison of muffle furnace and microwave heating methods. Powder Technology, 249, 234–240.
Geçgel, Ü., Kocabıyık, B., & Üner, O. (2015). Adsorptive removal of methylene blue from aqueous solution by the activated carbon obtained from the fruit of catalpa bignonioides. Water Air &Soil Pollution, 226(238), 1–14.
González, P. G., Hernández-Quiroz, T., & Garcia-González, L. (2014). The use of experimental design and response surface methodologies for the synthesis of chemically activated carbons produced from bamboo. Fuel Process Technology, 127, 133–139. doi:10.1016/j.fuproc.2014.05.035.
Gupta, S. S., & Bhattacharyya, K. G. (2011). Kinetics of adsorption of metal ions on inorganic materials: a review. Advances in Colloid and Interface Science, 162, 39–58.
Hamdaoui, O. (2006). Batch study of liquid-phase adsorption of methylene blue using cedar sawdust and crushed brick. Journal of Hazardous Materials, 135, 264–273.
Hameed, B. H., Tan, I. A. W., & Ahmad, A. L. (2008). Adsorption isotherm, kinetic modeling and mechanism of 2,4,6-trichlorophenol on coconut husk-based activated carbon. Chemical Engineering Journal, 144, 235–244.
Han, X., Chu, L., Liu, S., Chen, T., Ding, C., Yan, J., Cui, L., & Quan, G. (2015). Removal of methylene blue from aqueous solution using porous biochar obtained by KOH activation of peanut shell biochar. BioResources, 10(2), 2836–2849.
Ho, Y. S., & McKay, G. (1999). Pseudo-second order model for sorption processes. Process Biochemistry, 34, 451–465.
Islam, M. A., Tan, I. A. W., Benhouria, A., Asif, M., & Hameed, B. H. (2015). Mesoporous and adsorptive properties of palm date seed activated carbon prepared via sequential hydrothermal carbonization and sodium hydroxide activation. Chemical Engineering Journal, 270, 187–195.
Kasgoz, H., & Durmus, A. (2008). Dye removal by a novel hydrogel-clay nanocomposite with enhanced swelling properties. Polymers for Advanced Technologies, 19, 838–845.
Kavitha, D., & Namasivayam, C. (2007). Experimental and kinetic studies on methylene blue adsorption by coir pith carbon. Bioresource Technology, 98, 14–21.
Koca, I., Hasbay, I., Karadeniz, B., & Koca, A. F. (2014). Changes in the physicochemical and antioxidant characteristics of watermelon during pekmez production. Quality Assurance and Safety of Crops & Foods, 6(4), 411–418.
Lagergren, S. (1898). Zurtheorie der sogenannten adsorption gelösterstoffe. Kungliga Svenska Vetenskapsakademiens Handlingar, 24, 1–39.
Langmuir, I. (1916). The constitution and fundamental properties of solids and liquids. Journal of American Chemical Society, 38, 2221–2295.
Lee, Y.-W., & Park, J.-W. (2002). Adsorption characteristics of SO2 on activated carbon prepared from coconut shell with potassium hydroxide activation. Environmental Science & Technology, 36, 1086–1092.
Li, L., Liu, S., & Zhu, T. (2010). Application of activated carbon derived from scrap tires for adsorption of Rhodamine B. Journal of Environmental Sciences, 22, 1273–1280.
Liu, Y., Wang, J., Zheng, Y., & Wang, A. (2012). Adsorption of methylene blue by kapok fiber treated by sodium chlorite optimized with response surface methodology. Chemical Engineering Journal, 184, 248–255.
Lowell, S., & Shields, J. E. (1991). Powder surface area and porosity (3rd ed.). London: Chapman & Hall.
Mahmoodian, H., Moradi, O., Shariatzadeha, B., Salehf, T. A., Tyagi, I., Maity, A., Asif, M., & Gupta, V. K. (2015). Enhanced removal of methyl orange from aqueous solutions by poly HEMA-chitosan-MWCNT nano-composite. Journal of Molecular Liquids, 202, 189–198.
Malekbala, M. R., Khan, M. A., Hosseini, S., Abdullah, L. C., & Choong, T. S. Y. (2015). Adsorption/desorption of cationic dye on surfactant modified mesoporous carbon coated monolity: equilibrium, kinetic and thermodynamic studies. Journal of Industrial and Engineering Chemistry, 21, 369–377.
Martins, A. C., Pezoti, O., Cazetta, A. L., Bedin, K. C., Yamazaki, D. A. S., Bandoch, G. F. G., Asefa, T., Visentainer, J. V., & Almeida, V. T. (2015). Removal of tetracycline by NaOH-activated carbon produced from macadamia nut shells: kinetic and equilibrium studies. Chemical Engineering Journal, 260, 291–299.
Milonjić, S. K., Ruvarac, A. L., & Šušić, M. V. (1975). The heat of immersion of natural magnetite in aqueous solutions. ThermochimicaActa, 11, 261–266.
Njoku, V. O., Foo, K. Y., Asif, M., & Hameed, B. H. (2014). Preparation of activated carbons from rambutan (Nepheliumlappaceum) peel by microwave-induced KOH activation for acid yellow 17 dye adsorption. Chemical Engineering Journal, 222, 108–119. doi:10.1016/j.cej.2013.02.029.
Pezoti, O., Cazetta, A. L., Bedin, K. C., Souza, L. S., Martins, A. C., Silva, T. L., Santos Junior, O. O., Visentainer, J. V., & Almeida, V. C. (2016). NaOH-activated carbon of high surface area produced from guava seeds as a high-efficiency adsorbent for amoxicillin removal: kinetic, isotherm and thermodynamic studies. Chemical Engineering Journal, 288, 778–788.
Pezoti Junior, O., Cazetta, A. L., Gomes, R. C., Barizao, E. O., Souza, I. P. A. F., Martins, A. C., Asefa, T., & Almeida, V. C. (2014). Synthesis of ZnCl2-activated carbon from macadamia nut endocarp (Macadamia integrifolia) by microwave-assisted pyrolysis: optimization using RSM and methylene blue adsorption. Journal of Analytical and Applied Pyrolysis, 105, 166–176.
Przystas, W., Zablocka-Godlewska, E., & Grabinska-Sota, E. (2015). Efficacy of fungal decolorization of a mixture of dyes belonging to different classes. Brazilian Journal of Microbiology, 46(2), 415–424.
Rafatullah, M., Sulaiman, O., Hashim, R., & Ahmad, A. (2010). Adsorption of methylene blue on low-cost adsorbents: a review. Journal of Hazardous Materials, 177, 70–80.
Rahmani-Sani, A., Hosseini-Bandegharaei, A., Hosseini, S. H., Kharghani, K., Zarei, H., & Rastegar, A. (2015). Kinetic, equilibrium and thermodynamic studies on sorption of uranium and thorium from aqueous solutions by a selective impregnated resin containing carminic acid. Journal of Hazardous Materials, 286, 152–163.
Ramana, D. K. V., & Min, K. (2015). Activated carbon produced from pigeon peas hulls waste as a low-cost agro-waste adsorbent for Cu(II) and Cd(II) removal. Desalination and Water Treatment. doi:10.1080/19443994.2015.1013509.
Rashidzadeh, A., Olad, A., & Salari, D. (2015). The effective removal of methylene blue dye from aqueous solutions by NaAlg-g-poly(acrylic acid-co-acryl amide)/clinoptilolite hydrogel nanocomposite. Fibers and Polymers, 16(2), 354–362. doi:10.1007/s12221-015-0354-9.
Saleh, T. A., & Gupta, V. K. (2014). Processing methods, characteristics and adsorption behavior of tire derived carbons: a review. Advances in Colloid and Interface Science, 211, 93–101.
Sellin, R., Clacens, J.-M., & Coutanceau, C. (2010). A thermogravimetric analysis/mass spectroscopy study of the thermal and chemical stability of carbon in the Pt/C catalytic system. Carbon, 48, 2244–2254.
Sheha, R. R., & Metwally, E. (2007). Equilibrium isotherm modeling of cesium adsorption onto magnetic materials. Journal of Hazardous Materials, 143, 354–361.
Shi, L., Zhang, G., Wei, D., Yan, T., Xue, X., Shi, S., & Wei, Q. (2014). Preparation and utilization of anaerobic granular sludge-based biochar for the adsorption of methylene blue from aqueous solutions. Journal of Molecular Liquids, 198, 334–340.
Temkin, M. I., & Pyzhev, V. (1940). Kinetic of ammonia synthesis on promoted iron catalyst. Acta PhysChim USSR, 12, 327–356.
Üner, O., Geçgel, Ü., & Bayrak, Y. (2015). Preparation and characterization of mesoporous activated carbons from waste watermelon rind by using the chemical activation method with zinc chloride. Arabian Journal of Chemistry. doi:10.1016/j.arabjc.2015.12.004.
Weber, W. J., & Morris, J. C. (1963). Kinetic of adsorption on carbon from solutions. Journal of the Sanitary Engineering Division ASCE, 89, 31–60.
Wong, A., Koutsogiannis, Z., Greene, S., & McIntyre, S. (2013). A case of hemolysis and methemoglobinemia following amyl nitrite use in an individual with G6PD deficiency. Journal of Acute Medicine, 3, 23–25.
Yang, J., & Qiu, K. (2010). Preparation of activated carbon from walnut shell via vacuum chemical activation and their application for methylene blue removal. Chemical Engineering Journal, 165, 209–217.
Yener, J., Kopac, T., Dogu, G., & Dogu, T. (2008). Dynamic analysis of sorption of methylene blue on granular and powdered activated carbon. Chemical Engineering Journal, 144, 400–406.
Zhang, L., Song, X., Liu, X., Yang, L., Pan, F., & Lv, J. (2011). Studies on the removal of tetracycline by multi-walled carbon nanotubes. Chemical Engineering Journal, 178, 26–33.
Zhong, Z. Y., Yang, Q., Li, X. M., Luo, K., Liu, Y., & Zeng, G. M. (2012). Preparation of peanut hull-based activated carbon by microwave-induced phosphoric acid activation and its application in Remazol Brilliant Blue R adsorption. Industrial Crops and Products, 37(1), 178–185. doi:10.1016/j.indcrop.2011.12.015.
Acknowledgments
This study was funded by Trakya University Research Fund under the project number of TÜBAP 2014/130.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
● The adsorbent produced from Citrullus lanatus rind had high adsorption affinity and adsorption capacity of 231.48 mg/g for methylene blue dye.
● MB adsorption mechanism onto the adsorbent was explained.
● Intraparticle diffusion, film diffusion, and pore diffusion played significant role in MB adsorption.
● The best models were Langmuir model and pseudo-second-order model to describe equilibrium data and kinetic data, respectively.
● MB adsorption was physical, spontaneous, endothermic, and thermodynamically feasible.
Rights and permissions
About this article
Cite this article
Üner, O., Geçgel, Ü. & Bayrak, Y. Adsorption of Methylene Blue by an Efficient Activated Carbon Prepared from Citrullus lanatus Rind: Kinetic, Isotherm, Thermodynamic, and Mechanism Analysis. Water Air Soil Pollut 227, 247 (2016). https://doi.org/10.1007/s11270-016-2949-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-2949-1