Abstract
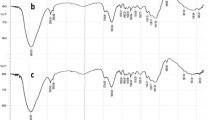
The removal of lead (II) and iron (III) from aqueous solutions using empty fruit bunch (EFB), oil palm leaves (OPL), oil palm frond (OPF), and oil palm bark (OPB) as biosorbents was investigated. The biosorbents were characterized through scanning electron microscopy, Brunauer–Emmett–Teller analysis, and Fourier transform infrared spectroscopy. Variables such as pH (2–12), biosorbent particle size (200–1,400 μm), adsorbent dosage (0.25–1.75 g/l), and agitation time (5–80 min) were investigated. The suitable pH range, particle size, adsorbent dosage, and agitation time for the removal of both metals were 5 to 6, 200 μm, 1 g/l, and 40 min, respectively. Under optimum conditions, OPB showed the highest adsorption efficiency of 80 % and 78 % for lead and iron, respectively. The adsorption equilibrium data were fitted to three adsorption isotherm models. The Langmuir isotherm showed the best result for both metals. The kinetics of the biosorption process was analyzed using pseudo-first-order and pseudo-second-order models. The latter showed a better fit for both metals. OPB biomass introduced the lowest chemical oxygen demand into the treated solution, with an average amount of 32.9 mg/l.












Similar content being viewed by others
References
Abia, A. A., & Asuquo, E. D. (2008). Sorption of Pb(II) and Cd(II) ions onto chemically unmodified and modified oil palm fruit fibre adsorbent: analysis of pseudo second order kinetic models. Indian Journal of Chemical Technology, 15, 341–348.
Aksu, Z. (2005). Application of biosorption for the removal of organic pollutants: a review. Process Biochemistry, 40, 997–1026.
Al-Rub, F. A. A. (2006). Biosorption of zinc on palm tree leaves: equilibrium, kinetics, and thermodynamics studies. Separation Science and Technology, 41, 3499–3515.
Amarasinghe, B. M. W. P. K., & Williams, R. A. (2007). Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater. Chemical Engineering Journal, 132, 299–309.
APHA, AWWA & WPCF. (2006). Standard methods for the examination of water and wastewater. USA: American Public Health Association.
Arslanoglu, H., Soner Altundogan, H., & Tumen, F. (2008). Preparation of cation exchanger from lemon and sorption of divalent heavy metals. Bioresource Technology, 99, 2699–2705.
Bhatnagar, A., Vilar, V. J. P., Botelho, C. M. S., & Boaventura, R. A. R. (2010). Coconut-based biosorbents for water treatment — a review of the recent literature. Advances in Colloid and Interface Science, 160, 1–15.
Bratskaya, S. Y., Pestov, A. V., Yatluk, Y. G., & Avramenko, V. A. (2009). Heavy metals removal by flocculation/precipitation using N-(2-carboxyethyl)chitosans. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 339, 140–144.
Bulgariu, D., & Bulgariu, L. (2012). Equilibrium and kinetics studies of heavy metal ions biosorption on green algae waste biomass. Bioresource Technology, 103, 489–493.
Chatterjee, S. K., Bhattacharjee, I., & Chandra, G. (2010). Biosorption of heavy metals from industrial waste water by Geobacillus thermodenitrificans. Journal of Hazardous Materials, 175, 117–125.
Chiew, Y. L., Iwata, T., & Shimada, S. (2011). System analysis for effective use of palm oil waste as energy resources. Biomass and Bioenergy, 35, 2925–2935.
Chu, K. H., & Hashim, M. A. (2002). Adsorption and desorption characteristics of zinc on ash particles derived from oil palm waste. Journal of Chemical Technology and Biotechnology, 77, 685–693.
Deniz, F., Karaman, S., & Saygideger, S. D. (2011). Biosorption of a model basic dye onto Pinus brutia Ten.: Evaluating of equilibrium, kinetic and thermodynamic data. Desalination, 270, 199–205.
Dogan, M., Abak, H., & Alkan, M. (2009). Adsorption of methylene blue onto hazelnut shell: kinetics, mechanism and activation parameters. Journal of Hazardous Materials, 164, 172–181.
El-Ashtoukhy, E. S. Z., Amin, N. K., & Abdelwahab, O. (2008). Removal of lead (II) and copper (II) from aqueous solution using pomegranate peel as a new adsorbent. Desalination, 223, 162–173.
Esposito, A., Pagnanelli, F., Lodi, A., Solisio, C., & Vegliò, F. (2001). Biosorption of heavy metals by Sphaerotilus natans: an equilibrium study at different pH and biomass concentrations. Hydrometallurgy, 60, 129–141.
Foo, K. Y., & Hameed, B. H. (2010). Insights into the modeling of adsorption isotherm systems. Chemical Engineering Journal, 156, 2–10.
Freeman, H. M. (Ed.). (1997). Standard handbook of hazardous waste treatment and disposal. New York: McGraw-Hill.
Ho, Y. S., & McKay, G. (1999). Pseudo-second order model for sorption processes. Process Biochemistry, 34, 451–465.
Jacques, R. A., Lima, E. C., Dias, S. L. P., Mazzocato, A. C., & Pavan, F. A. (2007). Yellow passion-fruit shell as biosorbent to remove Cr(III) and Pb(II) from aqueous solution. Separation and Purification Technology, 57, 193–198.
Le, X. T., Viel, P., Jégou, P., Sorin, A., & Palacin, S. (2009). Electrochemical-switchable polymer film: an emerging technique for treatment of metallic ion aqueous waste. Separation and Purification Technology, 69, 135–140.
Lesmana, S. O., Febriana, N., Soetaredjo, F. E., Sunarso, J., & Ismadji, S. (2009). Studies on potential applications of biomass for the separation of heavy metals from water and wastewater. Biochemical Engineering Journal, 44, 19–41.
Mansour, M. S., Ossman, M. E., & Farag, H. A. (2011). Removal of Cd (II) ion from waste water by adsorption onto polyaniline coated on sawdust. Desalination, 272, 301–305.
Mata, Y. N., Blázquez, M. L., Ballester, A., González, F., & Muñoz, J. A. (2009). Sugar-beet pulp pectin gels as biosorbent for heavy metals: preparation and determination of biosorption and desorption characteristics. Chemical Engineering Journal, 150, 289–301.
Miretzky, P., & Cirelli, A. F. (2009). Hg(II) removal from water by chitosan and chitosan derivatives: a review. Journal of Hazardous Materials, 167, 10–23.
Mohan, S., & Gandhimathi, R. (2009). Removal of heavy metal ions from municipal solid waste leachate using coal fly ash as an adsorbent. Journal of Hazardous Materials, 169, 351–359.
Nasef, M. M., & Yahaya, A. H. (2009). Adsorption of some heavy metal ions from aqueous solutions on Nafion 117 membrane. Desalination, 249, 677–681.
Nowak, B., Pessl, A., Aschenbrenner, P., Szentannai, P., Mattenberger, H., Rechberger, H., et al. (2010). Heavy metal removal from municipal solid waste fly ash by chlorination and thermal treatment. Journal of Hazardous Materials, 179, 323–331.
Ofomaja, A. E. (2010). Equilibrium studies of copper ion adsorption onto palm kernel fibre. Journal of Environmental Management, 91, 1491–1499.
Oh, S., Hassan, S., & Joo, J. (2009). Biosorption of heavy metals by lyophilized cells of Pseudomonas stutzeri. World Journal of Microbiology and Biotechnology, 25, 1771–1778.
Pelit, L., Ertaş, F. N., Eroĝlu, A. E., Shahwan, T., & Tural, H. (2011). Biosorption of Cu(II) and Pb(II) ions from aqueous solution by natural spider silk. Bioresource Technology, 102, 8807–8813.
Rao, R. A. K., & Khan, M. A. (2007). Removal and recovery of Cu(II), Cd(II) and Pb(II) ions from single and multimetal systems by batch and column operation on neem oil cake (NOC). Separation and Purification Technology, 57, 394–402.
Reddy, D. H. K., Ramana, D. K. V., Seshaiah, K., & Reddy, A. V. R. (2011). Biosorption of Ni(II) from aqueous phase by Moringa oleifera bark, a low cost biosorbent. Desalination, 268, 150–157.
Selvam, P. P., Preethi, S., Basakaralingam, P., Thinakaran, N., Sivasamy, A., & Sivanesan, S. (2008). Removal of rhodamine B from aqueous solution by adsorption onto sodium montmorillonite. Journal of Hazardous Materials, 155, 39–44.
Shpiner, R., Vathi, S., & Stuckey, D. C. (2009). Treatment of oil well "produced water" by waste stabilization ponds: removal of heavy metals. Water Research, 43, 4258–4268.
Southichak, B., Nakano, K., Nomura, M., Chiba, N., & Nishimura, O. (2009). Differences in adsorption mechanisms of heavy metal by two different plant biomasses: reed and brown seaweed. Water Science and Technology, 59, 339–346.
Sulaiman, O., Mohamad Amini, M.H., Rafatullah, M., Hashim, R. and Ahmad, A.: 2010, Adsorption equilibrium and thermodynamic studies of copper (II) ions from aqueous solutions by oil palm leaves, International Journal of Chemical Reactor Engineering 8, 1–17.
Tofighy, M. A., & Mohammadi, T. (2011). Adsorption of divalent heavy metal ions from water using carbon nanotube sheets. Journal of Hazardous Materials, 185, 140–147.
Tsekova, K., Todorova, D., Dencheva, V., & Ganeva, S. (2010). Biosorption of copper(II) and cadmium(II) from aqueous solutions by free and immobilized biomass of Aspergillus niger. Bioresource Technology, 101, 1727–1731.
Wahi, R., Ngaini, Z., & Jok, V. (2009). Removal of mercury, lead and copper from aqueous solution by activated carbon of palm oil fruit bunch. World Applied Sciences Journal, 5, 84–91.
Witek-Krowiak, A., Szafran, R. G., & Modelski, S. (2011). Biosorption of heavy metals from aqueous solutions onto peanut shell as a low-cost biosorbent. Desalination, 265, 126–134.
Xiong, Y., Chen, C., Gu, X., Biswas, B. K., Shan, W., Lou, Z., et al. (2011). Investigation on the removal of Mo(VI) from Mo-Re containing wastewater by chemically modified persimmon residua. Bioresource Technology, 102, 6857–6862.
Yacob, S., Ali Hassan, M., Shirai, Y., Wakisaka, M., & Subash, S. (2006). Baseline study of methane emission from anaerobic ponds of palm oil mill effluent treatment. Science of the Total Environment, 366, 187–196.
Yang, J., Wang, Q., Luo, Q., & Wu, T. (2009). Biosorption behavior of heavy metals in bioleaching process of MSWI fly ash by Aspergillus niger. Biochemical Engineering Journal, 46, 294–299.
Acknowledgments
The authors gratefully acknowledge the financial support from Universiti Sains Malaysia in the form of Postgraduate Research Grant Scheme (PRGS) (account no. 1001/PTEKIND/845001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khosravihaftkhany, S., Morad, N., Teng, T.T. et al. Biosorption of Pb(II) and Fe(III) from Aqueous Solutions Using Oil Palm Biomasses as Adsorbents. Water Air Soil Pollut 224, 1455 (2013). https://doi.org/10.1007/s11270-013-1455-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1455-y