Abstract
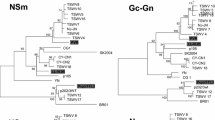
Resurgence of Tomato spotted wilt virus (TSWV) worldwide as well as in Hungary causing heavy economic losses directed the attention to the factors contributing to the outbreak of this serious epidemics. The introgression of Tsw resistance gene into various pepper cultivars seemed to solve TSWV control, but widely used resistant pepper cultivars bearing the same, unique resistance locus evoked the rapid emergence of resistance-breaking (RB) TSWV strains. In Hungary, the sporadic appearance of RB strains in pepper-producing region was first observed in 2010–2011, but in 2012 it was detected frequently. Previously, the non-structural protein (NSs) encoded by small RNA (S RNA) of TSWV was verified as the avirulence factor for Tsw resistance, therefore we analyzed the S RNA of the Hungarian RB and wild type (WT) isolates and compared to previously analyzed TSWV strains with RB properties from different geographical origins. Phylogenetic analysis demonstrated that the different RB strains had the closest relationship with the local WT isolates and there is no conserved mutation present in all the NSs genes of RB isolates from different geographical origins. According to these results, we concluded that the RB isolates evolved separately in geographic point of view, and also according to the RB mechanism.




Similar content being viewed by others
References
R. Goldbach, D. Peters, Semin. Virol. 5, 113–120 (1996)
J.A. Tomlinson, Ann. Appl. Biol. 110, 661–681 (1987)
D.E. Ullmann, T.L. German, J.L. Sherwood, D.M. Westcot, F.A. Cantone, Phytopathology 83, 456–463 (1993)
A.E. Whitfield, D.E. Ullmann, T.L. German, Ann. Rev. Phytopathol. 43, 459–489 (2005)
P. Roggero, V. Masenga, L. Tavella, Plant Dis. 86, 950–954 (2002)
P. Margaria, M. Ciuffo, M. Turina, Plant. Pathol. 53, 795 (2004)
F. Garcia-Arenal, B.A. McDonald, Phytopathology 93, 941–952 (2003)
M.L. Thomas-Carroll, R.A.C. Jones, Ann. Appl. Biol. 142, 235–243 (2003)
M. Sharman, D.M. Persley, Australas. Plant. Pathol. 35, 123–128 (2006)
J.H. Kim, G.S. Choi, J.S. Kim, C.K. Choi, Plant Pathol. J. 20, 297–301 (2004)
B.N. Chung, H.S. Choi, E.Y. Yang, J.D. Cho, I.S. Cho, G.S. Choi, S.K. Choi, Plant Pathol. J. 28, 87–92 (2012)
N.H. Hoang, H.-B. Yang, B.-C. Kang, Sci. Hortic. 161, 8–14 (2013)
T.L. German, D.E. Ullmann, J.W. Moyer, Ann. Rev. Phytopathol. 30, 315–348 (1992)
S. Adkins, Mol. Plant Pathol. 1, 151–157 (2000)
D. de Ronde, P. Butterbach, D. Lohuis, M. Hedil, J.W.M. van Lent, R. Kormelink, Mol. Plant Pathol. 14, 405–415 (2013)
D. de Ronde, A. Pasquier, S. Ying, P. Butterbach, D. Lohuis, R. Kormelink, Mol. Plant Pathol. 15, 185–195 (2014)
R. Gáborjányi, G. Csilléry, I. Tóbiás, G. Jenser, IXth Eucarpia Meeting, Budapest (1995), pp. 159–160
M.F. Clark, A.N. Adams, J. Gen. Virol. 34, 475–483 (1977)
S. Kumar, J. Dudley, M. Nei, K. Tamura, Brief Bioinform. 9, 299–306 (2008)
M. Jahn, I. Paran, K. Hoffmann, E.R. Radwanski, K.D. Livingstone, R.C. Grube, E. Aftergoot, M. Lapidot, J. Moyer, Mol. Plant Microbe Interact. 13, 673–682 (2000)
A. Takeda, K. Sugiyama, H. Nagano, M. Mori, M. Kaido, K. Mise, S. Tsuda, T. Okuno, FEBS Lett. 532, 75–79 (2002)
E. Bucher, T. Sijen, P. de Haan, R. Goldbach, M. Prins, J. Virol. 77, 1329–1336 (2003)
S. Sonoda, H. Tsumuki, Plant Sci. 166, 771–778 (2004)
P. Margaria, M. Ciuffo, D. Pacifico, M. Turina, Mol. Plant Microbe Interact. 20, 547–558 (2007)
F.A. Lovato, A.K. Inou-Nagata, T. Nagata, A.C. de Ávila, L.A. Pereira, R.O. Resende, Virus Res. 137, 245–252 (2008)
C. López, J. Aramburu, L. Galipienso, S. Soler, F. Nuez, L. Rubio, J. Gen. Virol. 92, 210–215 (2011)
D. Tentchev, E. Verdin, C. Marchal, M. Jacquet, J.M. Aguilar, B. Moury, J. Gen. Virol. 92, 961–973 (2011)
S.-Z. Pang, J.H. Bock, M.C. Gonsalves, J.L. Slightom, D. Gonsalves, Phytopathology 84, 243–249 (1994)
B.D. Harrison, Euphytica 124, 181–192 (2002)
M.R. Stevens, S.J. Scott, R.C. Gergerich, Euphytica 59, 9–17 (1992)
S. Roselló, M.J. Díez, F. Nuez, Eur. J. Plant Pathol. 104, 499–509 (1998)
J.J. Cho, D.M. Custer, S.H. Brommonschenkel, S.D. Tanksley, Acta Hortic. 431, 367–378 (1996)
G.J. Thompson, J.J.B. Van Zijl, Acta Hortic. 431, 379–384 (1996)
L.J. Latham, R.A.C. Jones, Ann. Appl. Biol. 133, 385–402 (1998)
J. Aramburu, M. Marti, Plant. Pathol. 52, 407 (2003)
M. Ciuffo, M.M. Finetti-Sialer, D. Gallitelli, M. Turina, Plant. Pathol. 54, 564 (2005)
K. Hoffmann, W.P. Qiu, J.W. Moyer, Mol. Plant Microbe Interact. 14, 242–249 (2001)
C. Hagen, A. Frizzi, J. Kao, L. Jia, M. Huang, Y. Zhang, S. Huang, Arch. Virol. 156, 1209–1216 (2011)
N. Mitter, V. Koundal, S. Williams, H. Pappu, PLoS One 8, e76276 (2013). doi:10.1371/journal.pone.0076276
R. Gáborjányi, G. Jenser, R. Vasdinyei, Hortic. Sci. 26, 91–94 (1994)
W.P. Qiu, S.M. Geske, C.M. Hickey, J.W. Moyer, Virology 244, 186–194 (1998)
S. Lian, J.-S. Lee, W.K. Cho, J. Yu, M.-K. Kim, H.-S. Choi, K.-H. Kim, PLoS One 8, e63380 (2013). doi:10.1371/journal.pone.0063380
A.C. Kaye, J.W. Moyer, E.J. Parks, I. Carbone, M.A. Cubeta, Phytopathology 101, 147–153 (2011)
M. Tsompana, J. Abad, M. Purugganan, W. Moyer, Mol. Ecol. 14, 53–66 (2005)
W. Qiu, J.W. Moyer, Phytopathology 89, 575–582 (1999)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11262_2014_1131_MOESM1_ESM.pdf
Online Resource 1 GenBank accession numbers and main information/characteristics of the TSWV strains implied in the molecular and phylogenetic analysis in this study. * RB: resistance-breaking; WT: wild type (PDF 256 kb)
Rights and permissions
About this article
Cite this article
Almási, A., Csilléry, G., Csömör, Z. et al. Phylogenetic analysis of Tomato spotted wilt virus (TSWV) NSs protein demonstrates the isolated emergence of resistance-breaking strains in pepper. Virus Genes 50, 71–78 (2015). https://doi.org/10.1007/s11262-014-1131-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-014-1131-3