Abstract
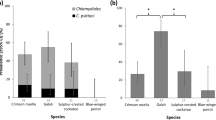
Immune reactivity for Chlamydophila (C.) psittaci in Slovenia was monitored in parrots, canaries, finches and nine species of recently captured free-living birds (house sparrows, Eurasian goldfinches, tree sparrows, chaffinches, European greenfinches, European serines, Eurasian siskins, Eurasian linnets and Eurasian bullfinches) in the period from 1991 to 2001. In subsequent years, specific IgG antibodies were found using immunofluorescence in parrots (0.7– 53.6%), canaries (0.0–3.5%), finches (0.0–5.7%) and in captured free-living birds (33.3% of Eurasian goldfinches in 1994). An experimental infection with C. psittaci was performed in order to study clinical signs and pathological changes in canaries and finches. The C. psittaci strain used for experimental infection was isolated from a cockatiel (Nymphicus hollandicus). Chlamydial DNA was extracted from clinical material followed by RFLP-PCR analysis. Infection of canaries and finches was confirmed in organ smears by direct immunofluorescence and a modified Gimenez staining method. In addition, serological tests of indirect immunofluorescence and complement fixation were applied. However, in spite of positive immunological reaction there were no clinical signs three weeks after infection. The present study includes results of a serological survey of persons belonging to the most important risk groups (breeders, pet shopkeepers and veterinarians). The results of microimmunofluorescence to identify the presence of specific antibodies and correlation between appearance of infection in birds and important risk groups are presented. Out of 143 persons belonging to the high-risk group we found 10 (7%) persons who were immunologically positive. Testing of two successive samples was used to demonstrate an increase in IgG and IgA titres in human sera. However, IgM, which is indicative of acute infection, could not be detected.
Similar content being viewed by others
REFERENCES
Anderson, I., Baxter, S.I. I., Dunbar, S., Rae, A.G., Philips, H.L., Clarkson, M.J. and Herring A.J., 1996. Analyses of the genomes of chlamydial isolates from ruminants and pigs support the adoption of the new species Chlamydia pecorum. International Journal of Systematic Bacteriology, 46, 24–51
Chahota, R., Katoch, R.C. and Joshi, V.B., 1997. Seroprevalence of Chlamydia psittaci in domestic poultry and wild birds. The Indian Journal of Poultry Science, 32, 67–71
Deutz A.F.K., Hinterdorfer, F. and Schuller, W., 1996. Serologische Untersuchung von Tierärzten auf Zoonosen. 1. Mitteilung: Grunddaten und Seropravalenzen gegenüber bakteriellen Zoonosen. Wiener Tierä rztliche Monatsschrift, 83, 283–288
Dorrestein, G.M., 1989. Chlamydiosis: a new approach in diagnosis and therapy. In: Proceedings of the Association of Avian Veterinarians, Munich; 1989:29–34
Dovč, A., 1998. Chlamydia psittaci infection in domestic and wild birds in Slovenia. Veterinarske novice, 24, 419–422
Duan, Y.J., Souriau, A., Mahe, A.M., Trap, D., Andersen, A.A. and Rodolakis, A., 1999. Serotyping of chlamydial clinical isolates from birds with monoclonal antibodies. Avian Diseases, 43, 22–28
Eugster, A.K., 1980. Handbook Series in Zoonoses. Section A, (CRC Press, Boca Raton)
Everett, K.D., Bush, R.M. and Andersen, A.A., 1999. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. Nov. and Simkaniaceae fam. Nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. International Journal of Systematic Bacteriology, 49, 415–440
Gaydos, C.A., Palmer, L., Quinn, T.C., Falkow, S. and Eiden, J.J., 1993. Phylogenetic relationship of Chlamydia pneumoniae to Chlamydia psittaci and Chlamydia trachomatis as determined by analysis of 16S ribosomal DNA sequences. International Journal of Systematic Bacteriology, 43, 610–612
Gosbell, I.B., Ross, A.D. and Turner, I.B., 1999. Chlamydia psittaci infection and reinfection in a veterinarian. Australian Veterinary Journal, 77, 511–513
Hinton, D.G., Shipley, A., Galvin, J.W., Harkin, J.T. and Brunton, R.A., 1993. Chlamydiosis in workers at a duck farm and processing plant. Australian Veterinary Journal, 70, 174–176
Huminer, D., Pitlik, S., Kitayin, D., Weissman, Y. and Samra, Z., 1992. Prevalence of Chlamydia psittaci infection among persons who work with birds. Israel Journal of Medical Sciences, 28, 739–741
Huminer, D., Samra, Z., Weisman, Y. and Pitlik, S., 1988. Family outbreaks of psittacosis in Israel. The Lancet, 2, 615–618
Janeczek, F., 1989. Chlamydia psittaci — diagnostic procedures in Psittaciformes: comparative studies on antigen detection in the cell culture and in the ELISA as well as antibody detection in the complement fixation and in blocking ELISA, (PhD thesis, University of Munich)
Kaleta, E.F. and Taday, E.M.A., 2003. Avian host range of Chlamydophila spp. based on isolation, antigens detection and serology. Avian Pathology, 32, 435–462
Kaltenboeck, B., Kousoulas, K.G. and Storz, J., 1991. Detection and strain differentiation of Chlamydia psittaci mediated by a two-step polymerase chain reaction. Journal of Clinical Microbiology, 29, 1969–1975
Ketz, C.J. and Carpenter, J.W., 1999. What is your diagnosis? Journal of Avian Medicine and Surgery, 13, 218–222
Kruszewicz, A.H. and Paszowska, K., 1992. Occurrence of Chlamydia psittaci in house sparrows (Passer domesticus). In: VIII. Tagung der Fachgruppe “Geflügelkrankheiten”, München; 1992: 124–129
Meijer, A., Morre, S.A., van den Brule, A.J., Savelkoul, P.H. and Ossewaarde, J.M., 1999. Genomic relatedness of Chlamydia isolates determined by amplified fragment length polymorphism analysis. Journal of Bacteriology, 181, 4469–4475
OIE, 1996. Manual of standards for diagnostic test and vaccines for list A and B diseases of mammals, birds and bees, (Office International des Epizooties, Paris)
Olsen, B., Persson, K. and Broholm, K.A., 1998. PCR detection of Chlamydia psittaci in faecal samples from passerine birds in Sweden. Epidemiology and Infection, 121, 481–483
Pearson, J.E., Gustafson G.A., Senne D.A. and Peterson L.A., 1989. Isolation and identification of Chlamydia psittaci from pet birds. Journal of the American Veterinary Medical Association, 195, 1564–1566
Pudjiatmoko, Fukushi, H., Ochiai, Y., Yamaguchi, T. and Hirai, K., 1997. Phylogenetic analysis of the genus Chlamydia based on 16S rRNA gene sequences. International Journal of Systematic Bacteriology, 47, 425–431
Rasmussen, S. and Timms, P., 1991. Detection of Chlamydia psittaci using DNA probes and the polymerase chain reaction. FEMS Microbiology Letters, 61, 169–173
Rodolakis, A. and Souriau, A., 1992. Restriction endonuclease analysis of DNA from ruminant Chlamydia psittaci and its relation to mouse virulence. Veterinary Microbiology, 31, 263–271
Sayada, C., Andersen, A.A., Storey, C., Milon, A., Eb. F., Hashimoto, N., Hirai, K., Elion, J. and Denamur, E., 1995. Usefulness of omp1 restriction mapping for avian Chlamydia psittaci isolate differentiation. Research in Microbiology, 146, 155–165
Simpson, V.R. and Bevan, R., 1989. Chlamydia psittaci infection in robins. The Veterinary Record, 21, 537
Vanrompay, D., Butaye, P., Sayada, C., Ducatelle, R. and Haesebrouck, F., 1997. Characterization of avian Chlamydia psittaci strains using omp1 restriction mapping and serovar-specific monoclonal antibodies. Research in Microbiology, 148, 327–333
Vanrompay, D., De Meurichy, W., Ducatelle, R. and Haesebrouck, F., 1994. Pneumonia in Moorish tortoises (Testudo graeca) associated with avian serovar A Chlamydia psittaci. The Veterinary Record, 135, 284–285
Vlahović, K., Župančić, Z., Gregurić, J. and Pavlak M., 1998. Zur Zuverlässigkeit diagnostischer Verfahren beim Nachweis von Infektionen mit Chlamydia spp. bei Vögein — Reliability of diagnostic methods in proving infections of Chlamydia spp. in birds. Zeitschrift für Jagdwissenschaft, 44, 133–139
Wilcke, B.W., Jr., Newcomer, C.E., Anver, M.R., Simmons, J.L. and Nace, G.W., 1983. Isolation of Chlamydia psittaci from naturally infected African clawed frogs (Xenopus laevis). Infection and Immunity, 41, 789–794
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dovc, A., Dovc, P., Kese, D. et al. Long-term Study of Chlamydophilosis in Slovenia. Vet Res Commun 29 (Suppl 1), 23–36 (2005). https://doi.org/10.1007/s11259-005-0834-2
Issue Date:
DOI: https://doi.org/10.1007/s11259-005-0834-2