Abstract
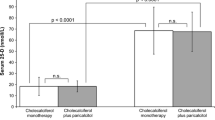
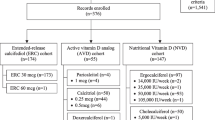
Nonselective vitamin D receptor activators (VDRA), such as calcitriol and alfacalcidol, have been successfully used in the treatment of secondary hyperparathyroidism (SHPT) in hemodialysis. Despite their beneficial effects on the control of serum PTH levels, their use has been limited by intolerance (development of hypercalcemia and hyperphosphatemia with consecutive cardiovascular toxicity). Apart from becoming intolerant, in 20–30 % of patients who use nonselective VDRA, serum PTH levels do not decrease appropriately despite increasing doses of these agents. These patients are considered calcitriol-resistant patients. Thus, calcitriol resistance and intolerance are two sides of the same coin: active vitamin D failure. Despite the clinical relevance of active vitamin D failure, definitions of resistance and intolerance are imprecise and have varied over time. More selective VDRA claim to produce less hypercalcemia and hyperphosphatemia and could help clinicians to overcome intolerance. Also, some studies have also shown that paricalcitol can be even useful in resistant patients. Significant limitations of iPTH as a reliable and useful clinical biomarker have been increasingly appreciated. There is evidence that intact PTH concentration must differ by 72 % between any two measurements before it can be considered a significant change. VDR polymorphisms could be involved in the development of SHPT in CKD patients. Interestingly, a higher incidence of the b allele of the VDR BsmI gene variant has been shown to be present in SHPT. The BsmI genotype can also affect the response of hemodialysis to IV calcitriol. A challenge for the future will be to establish biomarkers such as laboratory determinations or ultrasound findings that can help us to early identify those patients who will not respond appropriately to calcitriol or exhibit intolerable side effects .
Similar content being viewed by others
References
Slatopolsky E, Weerts C, Thielan J, Horst R, Harter H, Martin KJ (1984) Marked suppression of secondary hyperparathyroidism by intravenous administration of 1,25-dihydroxy-cholecalciferol in uremic patients. J Clin Invest 74(6):2136–2143
Brandi L (2008) 1alpha(OH)D3 One-alpha-hydroxy-cholecalciferol—an active vitamin D analog: clinical studies on prophylaxis and treatment of secondary hyperparathyroidism in uremic patients on chronic dialysis. Dan Med Bull 55(4):186–210
Brown AJ, Finch J, Slatopolsky E (2002) Differential effects of 19-nor-1, 25-dihydroxyvitamin D(2) and 1, 25-dihydroxyvitamin D(3) on intestinal calcium and phosphate transport. J Lab Clin Med 139(5):279–284
National Kidney Foundation (2003) K/DOQI Clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42:S1–S201
Llach F, Yudd M (2001) Paricalcitol in dialysis patients with calcitriol-resistant secondary hyperparathyroidism. Am J Kidney Dis 38(Suppl 5):S45–S50
Tonbul HZ, Solak Y, Atalay H, Turkmen K, Altintepe L (2012) Efficacy and tolerability of intravenous paricalcitol in calcitriol-resistant hemodialysis patients with secondary hyperparathyroidism: 12-month prospective study. Ren Fail 34(3):297–303
Capuano A, Serio V, Pota A, Memoli B, Andreucci VE (2009) Beneficial effects of better control of secondary hyperparathyroidism with paricalcitol in chronic dialysis patients. J Nephrol 22(1):59–68
Kazama JJ, Sato F, Omori K, Hama H, Yamamoto S, Maruyama H, Narita I, Gejyo F, Yamashita T, Fukumoto S, Fukagawa M (2005) Pretreatment serum FGF-23 levels predict the efficacy of calcitriol therapy in dialysis patients. Kidney Int 67(3):1120–1125
Vulpio C, Maresca G, Distasio E, Cacaci S, Panocchia N, Luciani G, Bossola M (2011) Switch from calcitriol to paricalcitol in secondary hyperparathyroidism of hemodialysis patients: responsiveness is related to parathyroid gland size. Hemodial Int 15(1):69–78
Ketteler M, Gross ML, Ritz E (2005) Calcification and cardiovascular problems in renal failure. Kidney Int Suppl 94:S120–S127
Kidney Disease: Improving Global Outcomes (KDIGO) CKD–MBD Work Group (2009) KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD–MBD). Kidney Int Suppl 113:S1–S130
Floege J, Kim J, Ireland E, Chazot C, Drueke T, de Francisco A, Kronenberg F, Marcelli D, Passlick-Deetjen J, Schernthaner G, Fouqueray B, Wheeler DC (2011) ARO Investigators Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant 26(6):1948–1955
Covic A, Kothawala P, Bernal M, Robbins S, Chalian A, Goldsmith D (2009) Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant 24(5):1506–1523
Barreto FC, Barreto DV, Moysés RM, Neves KR, Canziani ME, Draibe SA, Jorgetti V, Carvalho AB (2008) K/DOQI-recommended intact PTH levels do not prevent low-turnover bone disease in hemodialysis patients. Kidney Int 73(6):771–777
Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernán MA, Camargo CA Jr, Thadhani R (2005) Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol 16(4):1115–1125
Duranton F, Rodriguez-Ortiz ME, Duny Y, Rodriguez M, Daurès JP, Argilés A (2013) Vitamin D treatment and mortality in chronic kidney disease: a systematic review and meta-analysis. Am J Nephrol 37(3):239–248
Achinger SG, Mizani MR, Ayus JC (2010) Use of 3-hour daily hemodialysis and paricalcitol in patients with severe secondary hyperparathyroidism: a case series. Hemodial Int 14(2):193–199
Filiopoulos V, Malegos I, Stefanopoulou E, Patrikarea A (2007) Paricalcitol in dialysis patients with calcitriol—resistant secondary hyperparathyroidism. BANTAO Journal 5(2):70–73
Hansen D, Rasmussen K, Danielsen H, Meyer-Hofmann H, Bacevicius E, Lauridsen TG, Madsen JK, Tougaard BG, Marckmann P, Thye-Roenn P, Nielsen JE, Kreiner S, Brandi L (2011) No difference between alfacalcidol and paricalcitol in the treatment of secondary hyperparathyroidism in hemodialysis patients: a randomized crossover trial. Kidney Int 80(8):841–850
Raggi P, Chertow GM, Torres PU, Csiky B, Naso A, Nossuli K, Moustafa M, Goodman WG, Lopez N, Downey G, Dehmel B, Floege J, ADVANCE Study Group (2011) The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant 26(4):1327–1339
EVOLVE Trial Investigators, Chertow GM, Block GA, Correa-Rotter R, Drüeke TB, Floege J, Goodman WG, Herzog CA, Kubo Y, London GM, Mahaffey KW, Mix TC, Moe SM, Trotman ML, Wheeler DC, Parfrey PS (2012) Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 367(26):2482–2494
Drueke TB (2008) Is parathyroid hormone measurement useful for the diagnosis of renal bone disease? Kidney Int 73:674–676
Gardham C, Stevens PE, Delaney MP et al (2010) Variability of parathyroid hormone and other markers of bone mineral metabolism in patients receivinbg hemodialysis. Clin J Am Soc Nephrol 5:1261–1267
Sprague SM, Llach F, Amdahl M, Taccetta C, Batlle D (2003) Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int 63(4):1483–1490
Ureña P, Hruby M, Ferreira A, Ang KS, de Vernejoul MC (1996) Plasma total versus bone alkaline phosphatase as marker of bone turnover in hemodialysis patients. J Am Soc Nephrol 7:506–512
Fletcher S, Jones RG, Rayner HC et al (1997) Assessment of renal osteodystrophy in dialysis patients: use of bone alkaline phosphatase, bone mineral density and parathyroid ultrasound in comparison with bone histology. Nephron 75:412–419
Lehman G, Ott U, Kaemmerer D, Shuetze J, Wolf FG (2008) Bone histomorphometry and biochemical markers of bone turnover in patients with chronic kidney disease stages 3–5. Clin Nephrol 70:296–305
Coutteneye MM, D´Haese PC, Van Hoof VO et al (1996) Low serum levels of alkaline phosphatase of bone origin: a good marker of adynamic bone disease in haemodialysis patients. Nephrol Dial Transplant 11:1065–1072
Cavalier E, Delanaye P, Collette J et al (2006) Evaluation of different bone markers in haemodialysis patients. Clin Chim Acta 371:107–111
Reichel H, Esser A, Roth HJ, Schmidt-Gayk H (2003) Influence of PTH assay methodology on differential diagnosis of renal bone disease. Nephrol Dial Transplant 18:759–768
Sardiwal S, Gardham C, Coleman AE et al (2012) Bone-specific alkaline phosphatase concentrations are less variable than those of parathyroid hormone in stable hemodialysis patients. Kidney Int 82:100–105
Haussler MR, Whitfield GK, Haussler CA et al (1998) The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res 13(3):325–349
Nejentsev S, Godfrey L, Snook H et al (2004) Comparative high resolution analysis of linkage disequilibrium and tag single nucleotide polymorphisms between populations in the vitamin D receptor gene. Hum Mol Genet 13(15):1633–1639
Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP (2004) Genetics and biology of vitamin D receptor polymorphisms. Gene 338(2):143–156
Alvarez-Hernandez D, Naves-Diaz M, Gomez-Alonso C, Coto E, Cannata-Andia JB (2008) Tissue-specific effect of VDR gene polymorphisms on the response to calcitriol. J Nephrol 21(6):843–849
Valdivielso JM, Fernandez E (2006) Vitamin D receptor polymorphisms and diseases. Clin Chim Acta 371(1–2):1–12
Schmidt S, Chudek J, Karkoszka H et al (1997) The BsmI vitamin D-receptor polymorphism and secondary hyperparathyroidism. Nephrol Dial Transplant 12(8):1771–1772
Messa P, Sindici C, Cannella G et al (1998) Persistent secondary hyperparathyroidism after renal transplantation. Kidney Int 54(5):1704–1713
Fernández E, Fibla J, Betriu A, Piulats JM, Almirall J, Montoliu J (1997) Association between vitamin D receptor gene polymorphism and relative hypoparathyroidism in patients with chronic renal failure. J Am Soc Nephrol 8(10):1546–1552
Nagaba Y, Heishi M, Tazawa H, Tsukamoto Y, Kobayashi Y (1998) Vitamin D receptor gene polymorphisms affect secondary hyperparathyroidism in hemodialyzed patients. Am J Kidney Dis 32(3):464–469
Tagliabue J, Farina M, Imbasciati E, Vergani C, Annoni G (1999) BsmI polymorphism of the vitamin D receptor gene in hyperparathyroid or hypoparathyroid dialysis patients. Am J Clin Pathol 112(3):366–370
Marco MP, Martínez I, Amoedo ML et al (1999) Vitamin D receptor genotype influences parathyroid hormone and calcitriol levels in predialysis patients. Kidney Int 56(4):1349–1353
Marco MP, Martínez I, Betriu A, Craver L, Fibla MJ, Fernández E (2001) Influence of Bsml vitamin D receptor gene polymorphism on the response to a single bolus of calcitriol in hemodialysis patients. Clin Nephrol 56(2):111–116
Borràs M, Torregrossa V, Oliveras A et al (2003) BB genotype of the vitamin D receptor gene polymorphism postpones parathyroidectomy in hemodialysis patients. J Nephrol 16(1):116–120
Alvarez-Hernández D, Naves M, Santamaría I, Menárguez J, Torregrosa V, Cannata J (2003) Response of parathyroid glands to calcitriol in culture: Is this response mediated by the genetic polymorphisms in vitamin D receptor? Kidney Int Suppl 85:S19–S22
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Negri, A.L., Brandemburg, V.M. Calcitriol resistance in hemodialysis patients with secondary hyperparathyroidism. Int Urol Nephrol 46, 1145–1151 (2014). https://doi.org/10.1007/s11255-013-0637-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-013-0637-2