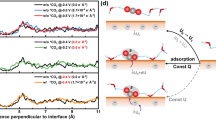
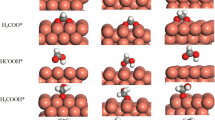
Abstract
The water–gas shift (WGS) reaction \( ({\text{CO}} + {\text{H}}_{ 2} {\text{O}} \to {\text{CO}}_{ 2} + {\text{H}}_{ 2} ) \) is a key process to the production of high purity H2 from gas streams rich in CO. The identification of the WGS reaction mechanism and the probable stable intermediates is critical to design the catalyst structure, optimize composition and tune reaction kinetics/thermodynamics to achieve the optimum selectivity and activity. In this study, first the WGS reaction steps on Cu(111) have been studied using X-ray photoelectron spectroscopy (XPS) and infrared reflection absorption spectroscopy under ultra-high vacuum (UHV) conditions. Then the interactions of H2O with CO on Cu(111) have been studied under elevated pressures (90 mTorr CO + 30 mTorr H2O) at 300–575 K with ambient pressure XPS. Under UHV conditions, non-dissociative adsorption of H2O on Cu(111) and Cu2O/Cu(111) was observed. Whereas H2O readily dissociates, by breaking the O–H bond on a chemisorbed O layer on Cu(111) to form OH species. Even though this OH interacts with adsorbed CO, it does not react to form any associative intermediate and simply desorbs as H2O at 275 K under UHV conditions. At ambient pressures, no associative intermediates species, only CO and OH, were observed in the reaction of CO with H2O although the catalytic production of H2 can be detected under these conditions. Since intermediate species other than CO and OH were not observed on Cu(111) under reaction conditions, we concluded that the redox mechanism is the dominant WGS pathway on Cu(111). The coupling of Cu to an oxide, Cu–CeO2 catalyst, or a carbide, Cu–TiC catalyst, favors an associative mechanism and produces a very large increase in the rate for the production of H2 through the WGS.









Similar content being viewed by others
References
Nakamura J, Campbell JM, Campbell CT (1990) Kinetics and mechanism of the water-gas shift reaction catalysed by the clean and Cs-promoted Cu(110) surface: a comparison with Cu(111). J Chem Soc, Faraday Trans 86(15):2725–2734
Oyama T (ed) (1996) Chemistry of metal carbides and nitrides. Springer, Berlin, p 536
Rodriguez JA, Ramirez PJ, Asara GG, Viñes F, Evans J, Liu P, Ricart JM, Illas F (2014) Charge polarization at a Au-TiC interface and the generation of highly active and selective catalysts for the low-temperature water-gas shift reaction. Angew Chem Int Ed 53(42):11270–11274. doi:10.1002/anie.201407208
Jakdetchai O, Nakajima T (2002) Mechanism of the water–gas shift reaction over Cu(110), Cu(111) and Cu(100) surfaces: an AM1-d study. J Mol Struc-Theochem 619(1–3):51–58
Lin C-H, Chen C-L, Wang J-H (2011) Mechanistic studies of water–gas-shift reaction on transition metals. J Phys Chem C 115(38):18582–18588
Tang Q-L, Chen Z-X, He X (2009) A theoretical study of the water gas shift reaction mechanism on Cu(111) model system. Surf Sci 603(13):2138–2144
Gokhale AA, Dumesic JA, Mavrikakis M (2008) On the mechanism of low-temperature water gas shift reaction on copper. J Am Chem Soc 130(4):1402–1414
Henderson MA (2002) The interaction of water with solid surfaces: fundamental aspects revisited. Surf Sci Rep 46(1–8):1–308
Thiel PA, Madey TE (1987) The interaction of water with solid surfaces: fundamental aspects. Surf Sci Rep 7(6–8):211–385
Rodriguez JA, Graciani J, Evans J, Park JB, Yang F, Stacchiola D, Senanayake SD, Ma S, Pérez M, Liu P, Sanz JF, Hrbek J (2009) Water-gas shift reaction on a highly active inverse CeOx/Cu(111) catalyst: unique role of ceria nanoparticles. Angew Chem Int Ed 48(43):8047–8050
Hinch BJ, Dubois LH (1992) Stable and metastable phases of water adsorbed on Cu(111). J Chem Phys 96(4):3262–3268
Yamamoto S, Andersson K, Bluhm H, Ketteler G, Starr DE, Schiros T, Ogasawara H, Pettersson LGM, Salmeron M, Nilsson A (2007) Hydroxyl-induced wetting of metals by water at near-ambient conditions. J Phys Chem C 111(22):7848–7850
Wang G-C, Nakamura J (2010) Structure sensitivity for forward and reverse water-gas shift reactions on copper surfaces: a DFT study. J Phy Chem Lett 1(20):3053–3057
Hollins P, Pritchard J (1979) Interactions of CO molecules adsorbed on Cu(111). Surf Sci 89(1–3):486–495
Senanayake SD, Mullins DR (2008) Redox pathways for HCOOH decomposition over CeO2 surfaces. J Phys Chem C 112(26):9744–9752
Hrbek J, Hoffmann FM, Park JB, Liu P, Stacchiola D, Hoo YS, Ma S, Nambu A, Rodriguez JA, White MG (2008) Adsorbate-driven morphological changes of a gold surface at low temperatures. J Am Chem Soc 130(51):17272–17273
Grass ME, Karlsson PG, Aksoy F, Lundqvist M, Wannberg B, Mun BS, Hussain Z, Liu Z (2010) New ambient pressure photoemission endstation at advanced light source beamline 9.3.2. Rev Sci Instrum 81(5):053106. doi:10.1063/1.3427218
Tillborg H, Nilsson A, Mårtensson N (1993) Shake-up and shake-off structures in core level photoemission spectra from adsorbates. J Electron Spectrosc Relat Phenom 62(1–2):73–93
Yang F, Choi Y, Liu P, Hrbek J, Rodriguez JA (2010) Autocatalytic reduction of a Cu2O/Cu(111) surface by CO: sTM, XPS, and DFT studies. J Phys Chem C 114(40):17042–17050
Senanayake SD, Stacchiola D, Evans J, Estrella M, Barrio L, Pérez M, Hrbek J, Rodriguez JA (2010) Probing the reaction intermediates for the water–gas shift over inverse CeOx/Au(111) catalysts. J Catal 271(2):392–400
Senanayake SD, Waterhouse GIN, Chan ASY, Madey TE, Mullins DR, Idriss H (2007) The reactions of water vapour on the surfaces of stoichiometric and reduced uranium dioxide: a high resolution XPS study. Catal Today 120(2):151–157
Hoffmann FM (1983) Infrared reflection-absorption spectroscopy of adsorbed molecules. Surf Sci Rep 3(2–3):107–192
Mudiyanselage K, Senanayake SD, Feria L et al (2013) Importance of the metal-oxide interface in catalysis: in situ studies of the water-gas shift reaction by ambient-pressure X-ray photoelectron spectroscopy. Angew Chem Int Ed 52:5101–5105
Rodriguez JA, Illas F (2012) Activation of noble metals on metal-carbide surfaces: novel catalysts for CO oxidation, desulfurization and hydrogenation reactions. Phys Chem Chem Phys 14:427–438
Rodriguez JA, Evans J, Feria L, Vidal AB, Liu P, Nakamura K, Illas F (2013) CO2 hydrogenation on Au/TiC, Cu/TiC and Ni/TiC: production of CO, methane and methanol. J Catal 307:162–169
Moon DJ, Ryu JW (2004) Molybdenum carbide water-gas shift catalyst for fuel cell-powered vehicles applications. Catal Lett 92(1–2):17–24
Acknowledgments
The work at BNL (Chemistry Department and National Synchrotron Light Source) was financed by the US Department of Energy (DOE), Office of Basic Energy Science (DE-AC02-98CH10086). INTEVEP and IDB financed the work done at UCV. The AP-XPS spectra were acquired at the Advanced Light Source (beamline 9.3.2), which is supported by the US DOE under contract no. DE-AC02-05CH11231.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mudiyanselage, K., Senanayake, S.D., Ramirez, P.J. et al. Intermediates Arising from the Water–Gas Shift Reaction over Cu Surfaces: From UHV to Near Atmospheric Pressures. Top Catal 58, 271–280 (2015). https://doi.org/10.1007/s11244-015-0368-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-015-0368-y