Abstract
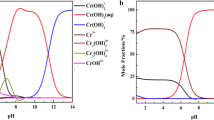
The oxidation of isosorbide (S) by chromic acid (CrVI) has been studied in aqueous perchlorate solution at a constant ionic strength of 3.0 mol dm−3 and temperature of 25 °C. The course of the reaction was followed spectrophotometrically. The reaction exhibited first-order dependence on [CrVI], less than unit order with respect to [S], and fractional-second-order dependence with respect to [H+]. Variation of the ionic strength or dielectric constant of the medium had no significant effects on the oxidation rate. Addition of MnII inhibited the oxidation rate. The oxidation product of isosorbide was identified as the corresponding monoketone derivative, namely (1S,4S,5R)-4-hydroxy-2,6-dioxabicyclo[3.3.0] octan-8-one. A mechanism for the oxidation is proposed, and the corresponding rate-law expression has been deduced. The activation parameters associated with the second-order rate constant are presented and discussed.









Similar content being viewed by others
References
Rose M, Palkovits R (2012) ChemSusChem 5:167–176
Flèche G, Huchette M (1986) Starch/Stärke 38:26–30
Lee C-H, Takagi H, Okamoto H, Kato M (2015) Polym J 47:639–643
Pfützenreuter R, Helmin M, Palkovits S, Palkovits R, Rose M (2014) Catal Today 234:113–118
Chatti S, Schwarz G, Kricheldorf HR (2006) Macromolecules 39:9064–9070
Van Buu ON, Aupoix A, Doan THN, Vo-Thanh G (2009) New J Chem 33:2060–2072
Van Buu ON, Vo-Thanh G (2007) Lett Org Chem 4:158–167
Kumar S, Ramachandran U (2005) Tetrahedron 61:4141–4148
Paolucci C, Rosini G (2007) Tetrahedron Asymmetry 18:2923–2946
Liu FW, Yan L, Zhang JY, Liu HM (2006) Carbohydr Res 341:332–338
Bersot JC, Jacquel N, Saint-Loup R, Fuertes P, Rousseau A, Pascault JP, Spitz R, Fenouillot F, Monteil V (2011) Macromol Chem Phys 212:2114–2120
Carcedo C, Dervisi A, Fallis AI, Ooi L, Malik AKM (2004) Chem Commun 10:1236–1237
Vazifehasl Z, Hemmati S, Zamanloo M, Jaymand M (2013) Macromol Res 21:427–434
Chimatadar SA, Koujalagi SB, Nandibewoor ST (2001) Transit Met Chem 26:662–667
Sen Gupta KK, Chakladar JK (1974) J Chem Soc, Dalton Trans 2:222–225
Manhas MS, Kumar P, Mohamed F, Khan Z (2008) Coll Surf A 320:240–246
Odebunmi EO, Obike AI, Owalude SO (2009) Int J Biol Chem Sci 3:178–185
Fawzy A, Ashour SS, Musleh MA, Hassan RM, Asghar BH (2016) J Saudi Chem Soc 20:450–458
Hassan RM, Ahmed SM, Fawzy A, Abdel-Kader DA, Ikeda Y, Takagi HD (2010) Cat Commun 11:611–615
Fawzy A, Altass HM (2016) Transit Met Chem 41:115–124
Barnhart J (1997) J Soil Contam 6:561–568
Costa M (1997) Crit Rev Toxicol 27:431–442
Jacquet F, Rigal L, Gaset A (1990) J Chem Technol Biotechnol 48:493–506
Jacquet F, Audinos R, Delmas M, Gaset A (1985) Biomass 6:193–209
Gross J, Tauber K, Fuchs M, Schmidt NG, Rajagopalan A, Faber K, Fabian WMF, Pfeffer J, Haasb T, Kroutil W (2014) Green Chem 16:2117–2121
Furniss BS, Hannaford AJ, Smith WG, Tatchell AR (2004) In Vogel’s textbook of practical organic chemistry, 5th edn. Pearson Education Ltd
Espenson JH, King EL (1963) J Am Chem Soc 85:3328–3333
Sen Gupta KK, Sarkar T (1975) Tetrahedron 31:123–127
Bose RN, Moghaddas B, Gelerinter E (1992) Inorg Chem 31:1987–1994
Milazzo G, Caroli S, Sharma VK (1978) Tables of standard electrode metal potentials. Wiley, New York
Bailey N, Carrington A, Lott KAK, Symons MCR (1960) J Chem Soc 290–297
Michaelis L, Menten ML (1913) Biochem Z 49:333–369
Frost AA, Person RG (1971) Kinetics and mechanism. Wiley Eastern, New Delhi, p 147
Rochester CH (1970) Progress in reaction kinetics. Pergamon Press, Oxford
Weissbekger A (1974) Investigation of rates and mechanism of reactions in techniques of chemistry, Wiley, p 421
Acknowledgements
We thank the department of Chemistry, Umm Al-Qura University for the use of all instrumentation facilities and Prof. M. Majdoub for discussions.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Fawzy, A., Guesmi, N.E., Althagafi, I.I. et al. A study of the kinetics and mechanism of chromic acid oxidation of isosorbide, a chiral biomass-derived substrate, in aqueous perchlorate solution. Transit Met Chem 42, 229–236 (2017). https://doi.org/10.1007/s11243-017-0126-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-017-0126-z