Abstract
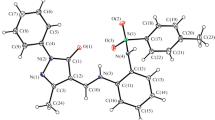
A Schiff base, N,N′-(3,4-dithiahexane-1,6-diyl)bis(5-methylsalicylideneimine), was synthesized and characterized by X-ray crystallography. Dimeric complexes of nickel(II), palladium(II), and vanadium(IV) were synthesized by the reactions of the Schiff base with nickel(II) acetate, palladium(II) acetate, and vanadyl acetylacetonate in 1:1 molar ratio. In all three complexes, the thiol group was deprotonated and coordinated to the metal. The X-ray structure of the Schiff base showed that in the crystalline form, the SH groups were oxidized to the corresponding disulfide. In the dimeric complexes, coordination took place through the azomethine nitrogen, enolic oxygen, and sulfur atoms. The metal-to-ligand ratio was 1:1, and molar conductance data revealed that the metal complexes were nonelectrolytes. The free Schiff base and its complexes showed photoluminescence in methanol at room temperature. The redox behavior of the compounds was studied by cyclic voltammetry in DMF, which showed both quasi-reversible and irreversible processes. The interaction of the complexes with DNA was investigated by electronic absorption spectroscopy.










Similar content being viewed by others
References
Utreja D, Singh S, Kaur M (2015) Curr Bioact Compd 11:215–230
Mishra AP, Khare M (2000) J Indian Chem Soc 77:367–370
Pinedo HM, Schornagel JM (1996) Platinum and other metal coordination compounds in cancer chemotherapy, vol 2. Plenum Press, New York
Costamagna JV, Larorre R, Alvarado A, Mena G (1992) Coord Chem Rev 119:67–88
Saleh AA (2005) J Coord Chem 58:255–270
Zhuang ZJ, Okawa H, Matsumoto N, Sakiyama H, Kida SJ (1991) J Chem Soc, Dalton Trans 497–500
Nelkenbaum E, Kapon M, Eisen MS (2005) Organometallics 24:2645–2659
Tabassum S, Al-Asbahy WM, Afzal M, Shamsi M, Arjmand F (2012) J Lumin 132:3058–3065
Lippert B (2000) Coord Chem Rev 200:487–516
Mudasir E, Wijaya K, Wahyuni ET, Inoue H, Yoshioka N (2007) Spectrochim Acta A 66:163–170
Shafaatian B, Soleymanpour A, Kholghioskouei N, Notash B, Rezvani SA (2014) Spectrochim Acta A 128:363–369
Shafaatian B, Ozbakzaei Z, Notash B, Rezvani SA (2015) Spectrochim Acta A 140:248–255
Shafaatian B, Hashemibagha M, Notash B, Rezvani SA (2015) J Organomet Chem 791:51–57
Gupta Bhowon M, Jhaumeer-Laulloo S, Soukhee N, Allibacus A, Shiboo V (2007) J Coord Chem 60:1335–1343
Stoe & Cie (2005) X-AREA: program for the acquisition and analysis of data, version 1.30. Stoe & Cie GmbH, Darmatadt
Stoe & Cie (2005) X-RED: program for data reduction and absorption correction, version 1.28b. Stoe & Cie GmbH, Darmatadt
Stoe & Cie (2004) X-SHAPE: program for crystal optimization for numerical absorption correction, version 2.05. Stoe & Cie GmbH, Darmatadt
Sheldrick GM (2008) Acta Cryst A A64:112–122
Stoe & Cie (2000) X-STEP32: crystallographic package, version 1.07b. Stoe & Cie GmbH, Darmatadt
Marmur J (1961) J Mol Biol 3:208–218
Constable EC, Housecroft CE, Zampese JA (2011) Inorg Chem Commun 14:1703–1705
Casellato U, Tamburini S, Tomasin P, Vigato PA (2002) Inorg Chim Acta 341:118–126
Abu-Hussen AAA (2006) J Coord Chem 59:157–176
Mathew M, Gary AJ, Palenik GJ (1972) J Am Chem Soc 92:3197–3198
Krishnankutty K, Michael J (1991) J Coord Chem 22:327–330
Silverstein M, Bassler GC, Morril TC (1991) Spectrometric identification of organic compounds. Wiley, New York
Basak S, Sen S, Banerjee S, Mitra S, Rosair G, Rodriguez MTG (2007) Polyhedron 26:5104–5112
Chen Y, Zhao X-J, Gan X, Fu W-F (2008) Inorg Chim Acta 361:2335–2342
Gomes L, Periera ES, De Castro B (2000) J Chem Soc, Dalton Trans 1373–1379
Geary WJ (1971) Coord Chem Rev 7:81–122
Tsuchimoto M, Hoshina G, Yoshioka N (2000) J Solid State Chem 153:9–15
Heng MP, Sinniah SK, Teoh WY, Sim KS, Cheah YQ, Tan KW, Ng SW, Cheah YK, Tan KW (2015) Spectrochim Acta A 150:360–372
Patel MN, Patel CR, Joshi HN (2012) Spectrochim Acta A 97:66–73
Gökçe C, Gup R (2013) J Photochem Photobiol B Biol 122:15–23
Jing B, Dong J, Li J, Xu T, Li L (2013) J Coord Chem 66:520–529
Peng B, Chao H, Sun B, Li H, Gao F, Ji L-N (2007) J Inorg Biochem 101:404–411
Pothiraj K, Baskaran T, Raman N (2012) J Coord Chem 65:2110–2126
Acknowledgments
We gratefully acknowledge the support of this work by Damghan University Research Council.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix: Supplementary data
Appendix: Supplementary data
CCDC 1053368 contains the supplementary crystallographic data for compound A. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44)1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk.
Rights and permissions
About this article
Cite this article
Mohammadtabar, F., Shafaatian, B., Soleymanpour, A. et al. Synthesis, spectral characterization, X-ray crystal structure, electrochemical studies, and DNA interactions of a Schiff base pro-ligand and its homobimetallic complexes containing the cysteamine moiety. Transition Met Chem 41, 475–484 (2016). https://doi.org/10.1007/s11243-016-0043-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-016-0043-6