Abstract
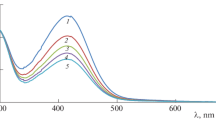
The complex [RuIII(edta)(H2O)]− (edta4− = ethylenediaminetetraacetate) catalyzes the oxidation of thiourea (TU) by peroxomonosulfate ion (HSO5 −). The kinetics of the catalytic oxidation process was studied by using stopped-flow and rapid-scan spectrophotometry as a function of [RuIII(edta)(H2O)−], [HSO5 −] and [TU] at a constant pH of 6.2 (phosphate buffer) and temperature of 25 °C. Spectral and kinetic data are suggestive of a catalytic pathway involving rapid formation of a [RuIII(edta)(TU)]− intermediate complex by reaction of [RuIII(edta)(H2O)]− with TU, followed by the oxidation of the coordinated TU in which HSO5 − reacts directly with the S atom of TU coordinated to the RuIII(edta) complex. Analysis of the reaction mixture at the end of the reaction revealed the formation of formamidine disulfide (TU2) as a major product; however, thiourea dioxide (TUO2) and sulfate were also observed if the reaction mixture was kept for longer time periods. A detailed mechanism in agreement with the spectral and kinetic data is presented.




Similar content being viewed by others
References
Curtis WE, Muldrow ME, Parker NB, Barkley R, Linas SL, Repine JE (1988) Proc Natl Acad Sci 85:3422
Sprong RC, Aarsman CJM, van Oirschot JFLM, van Asbeck BS (1997) J Lab Clin Med 129:470
Kelner MJ, Bagnell R, Welch KJ (1990) J Biol Chem 265:1306
Lai Y-L, Wu H-D, Chen CF (1998) J Cardiovasc Pharmacol 32:714
Wang W, Schuchmann MN, Schuchmann H-P, Knolle W, von Sonntag J, von Sonntag C (1999) J Am Chem Soc 121:238
Simoyi RH, Epstein IR, Kustin K (1994) J Phys Chem 98:551
Sharma V, Joshi WV, Millero FJ, Connor D (1999) Environ Sci Technol 33:2645
Kudrik EV, Theodoridis AK, van Eldik R, Makarov SV (2005) Dalton Trans 6:1117–1122
Henry NPO, Harutyuneran KY, Byrd JE (1979) Inorg Chem 18:197
Chatterjee D, Paul B, Mukherjee R (2013) Dalton Trans 42:10056–10060
Baly J (1986) J Electroanal Chem 214:481–483
Diamantis AA, Dubrwaski JV (1981) Inorg Chem 20:1142
Matsubara T, Creutz C (1979) Inorg Chem 18:1956–1966
Bajaj HC, van Eldik R (1988) Inorg Chem 27:4052–4055
Chatterjee D, Sikdar A, Patnam VR, Theodoridis A, van Eldik R (2008) Dalton Trans 29:3851–3856
Gao Q, Wang G, Sun Y, Epstein IR (2008) J Phys Chem A 112:5771
Acknowledgments
Papiya Sarkar thanks CSIR, New Delhi, for junior research fellowship (CSIR-JRF).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sarkar, P., Chatterjee, D. Oxidation of thiourea by peroxomonosulfate ion catalyzed by a ruthenium(III) complex: kinetic and mechanistic studies. Transition Met Chem 41, 9–13 (2016). https://doi.org/10.1007/s11243-015-9991-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-015-9991-5