Abstract
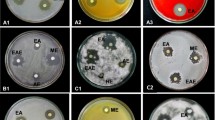
Herbal medicine requires searching for new sources with antimicrobial activity. Alkanna sp. (Boraginaceae) is widely used in medicine due to detoxification and antibacterial effects. The aim of this study was to obtain Alkanna orientalis (L.) Boiss. plant callus extracts, to investigate antimicrobial activity of extracts against bacteria and yeast and to reveal responsible mechanisms. Callus tissue cultures have been obtained using different nutrient media. Antimicrobial activity and minimal inhibitory concentrations (MIC) were determined by the methods of extract diffusion in agar and dilution using different test-microorganisms. Quantity and quality of naphthoquinones were determined using spectrophotometric and high performance liquid chromatographic analyses. H+/K+ exchange by whole cells was assayed using selective electrodes, ATPase activity and SH-groups number of membrane vesicles—by spectrophotometric methods. It was revealed that callus extracts containing naphthoquinones (0.19 ± 0.01 %) possessed bacteriostatic activity against gram-positive bacteria including pathogenic ones (MIC were 125–750 μg/mL−1), and bactericidal activity against lactic acid bacteria (Lactobacillus acidophilus, L. rhamnosus) (MIC was 250 μg/mL). MIC against Enterococcus hirae was 250 μg/mL of callus extract or 31.25 μg/mL of shikonin. It depended on the medium content, cultivation duration and the activity remained for 10–11 years. Moreover, intact root extracts inhibited H+/K+ exchange of E. hirae but callus extracts had a stronger effect. The inhibitor, N,N′-dicyclohexylcarbodiimide (DCCD)-sensitive H+/K+ exchange was changed; ATPase activity and SH-groups number were lowered by two and more fold under the influence of the extracts. Alkanet root and callus tissues extracts were concluded to have a high antimicrobial activity. So, they might directly affect the FOF1-ATPase which in turn regulates the bacterial growth. These results are useful for further investigation of alkanet extracts composition and their application as an alternative antimicrobial agent in pharmaceutical and food industry as well as in medicine.




Similar content being viewed by others
Abbreviations
- MS:
-
Murashige and Skoog
- BAP:
-
6-Benzylaminopurine
- DCCD:
-
N,N′-dicyclohexylcarbodiimide
- FOF1 :
-
Proton-translocating ATPase
- IAA:
-
Indole-3-acetic acid
- ∆µ +H :
-
Proton-motive force
- MEP:
-
Multidrug efflux pump
- MIC:
-
Minimal inhibitory concentration
References
Abdallah EM (2011) Plants: an alternative source for antimicrobials. J Appl Pharm Sci 1(1):16–20
Bame JR, Graf TN, Junio HA, Bussey RO 3rd, Jarmusch SA, El-Elimat T, Falkinham JO 3rd, Oberlies NH, Cech RA, Cech NB (2013) Sarothrin from Alkanna orientalis is an antimicrobial agent and efflux pump inhibitor. Planta Med 79(5):327–329
Baronetz NG, Adlova GP, Melnikova VA (2001) Influence of extracts of medicinal plants on the growth of microorganisms. J Microbiol Epidemiol Immunol 5:71–72 (in Russian)
Benson HJ (2002) Microbiological applications, 8th edn. McGraw Hill, New York, p 87
Calo JR, Crandall PG, O’Bryan CA, Ricke SC (2015) Essential oils as antimicrobials in food systems—a review. Food Control 54:111–119
Chen X, Oppenheim J, Howard OM (2001) Shikonin, a component of antiinflammatory Chinese herbal medicine, selectively blocks chemokine binding to CC chemokine receptor-1. Int Immunopharmacol 1:229–236
Chen X, Yang L, Zhang N, Turpin JA, Buckheit RW, Osterling C, Oppenheim JJ, Howard OM (2003) Shikonin, a component of Chinese herbal medicine, inhibits chemokine receptor function and suppresses human immunodeficiency virus type 1. Antimicrob Agents Chemother 47(9):2810–2816
Cook GM, Greening C, Hards K, Berney M (2014) Energetics of pathogenic bacteria and opportunities for drug development. Adv Microb Physiol 65:1–62
Dadi P, Ahmad M, Ahmad Z (2009) Inhibition of ATPase activity of Escherichia coli ATP synthase by polyphenols. Int J Biol Macromol 45(1):72–79
Darbinian-Sarkissian N, Darbinyan A, Otte J, Radhakrishnan S, Sawaya BE, Arzumanyan A, Chipitsyna G, Popov Y, Rappaport J, Amini S, Khalili K (2006) p27SJ, a novel protein in St John’s Wort, that suppresses expression of HIV-1 genome. Gene Ther 13(4):288–295
Fiamegos YC, Kastritis PL, Exarchou V, Han H, Bonvin AM, Vervoort J, Lewis K, Hamblin MR, Tegos GP (2011) Antimicrobial and efflux pump inhibitory activity of caffeoylquinic acids from Artemisia absinthium against Gram-positive pathogenic bacteria. PLoS One 6(4):e18127
Gafner S, Wolfender JL, Nianga M, Stoeckli-Evans H, Hostettmann K (1996) Antifungal and antibacterial naphthoquinones from Newbouldialaevis roots. Phytochemistry 42(5):1315–1320
Gharehmatrossian S, Popov Y, Ghorbanli M, Safaeian Sh (2012) Antioxidant activities and cytotoxic effects of whole plant and isolated culture of Artemisia aucheri Boiss. Asian J Pharmaceut Clin Res 5(4):95–98
Gibbons S (2008) Phytochemicals for bacterial resistance—strengths, weaknesses and opportunities. Planta Med 74(6):594–602
Hussain A, Qarshi IA, Nazir H, Ullah I (2012) Plant tissue culture: current status and opportunities. [Online]. http://www.intechopen.com/books/recent-advances-in-plant-invitro-culture/plant-tissue-culture-current-status-and-opportunities/doi/10.5772/50568
Kakinuma Y (1998) Inorganic cation transport and energy transduction in Enterococcus hirae and other Streptococci. Microbiol Mol Biol Rev 62(4):1021–1045
Malik S, Bhushan S, Sharma M, Ahuja PS (2014) Biotechnological approaches to the production of shikonins: a critical review with recent updates. Crit Rev Biotechnol. doi:10.3109/07388551.2014.961003
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue culture. Physiol Plantarum 15(3):473–479
Nagata K, Hirai KI, Koyama J, Wada Y, Tamura T (1998) Antimicrobial activity of novel furanonaphthoquinone analogs. Antimicrob Agents Chemother 42(3):700–702
Oktyabrsky O, Vysochina G, Muzyka N, Samoilova Z, Kukushkina T, Smirnova G (2009) Assessment of anti-oxidant activity of plant extracts using microbial test systems. J Appl Microbiol 106(4):1175–1183
Poladyan A, Trchounian A (2006) The increase in the number of accessible SH groups in the Enterococcal membrane vesicles by ATP and nicotinamide adenine dinucleotides. Curr Microbiol 52(4):300–304
Poladyan A, Trchounian A (2011) Transport of protons and potassium ions through the membranes of bacteria Enterococcus hirae dependent on ATP and nicotinamide adenine dinucleotides. Biophysics 56(4):668–671
Poladyan A, Kirakosyan G, Trchounian A (2006) Growth and proton–potassium exchange in the bacterium Enterococcus hirae: the effect of protonofore and the role of redox potential. Biophysics 51(3):447–451
Sasaki K, Abe H, Yoshizaki F (2002) In vitro antifungal activity of naphthoquinone derivatives. Biol Pharm Bull 25(5):669–670
Schempp C, Pelz K, Wittmer A (1999) Antibacterial activity of hyperforin from St. John’s wort, against multiresistant Staphylococcus aureus and Gram-positive bacteria. Lancet 353:2129
Scorzoni L, Benaducci T, Almeida AMF, Silva DHS, Bolzani VS, Mendes-Giannini MJS (2007) Comparative study of disk diffusion and microdilution methods for evaluation of antifungal activity of natural compounds against medical yeasts Candida spp and Cryptococcus sp. Rev Ciên Farm Básica Appl 28(1):25–34
Shen CC, Syu WJ, Li SY, Lin CH, Lee GH, Sun CM (2002) Antimicrobial activities of naphthazarins from Arnebiaeuchroma. J Nat Prod 65(12):1857–1862
Stavri M, Piddock L, Gibbons S (2007) Bacterial efflux pumps inhibitors from natural sources. J Antimicrob Chemother 59(6):1247–1260
Sykłowska-Baranek K, Pietrosiuk A, Gawron A, Kawiak A, Łojkowska E, Jeziorek M, Chinou I (2012) Enhanced production of antitumour naphthoquinones in transgenic hairy root lines of Lithospermum canescens. PCTOC J Plant Biotechnol 108(2):213–219
Tabata M, Fujita Y (1985) Production of shikonin by plant cell cultures. In: Zaitlin M, Day P, Hollaender A, Wilson CM (eds) Biotechnology in plant science: relevance to agriculture in the eighties. FL, Academic Press, New York, Orlando, pp 207–218
Tabata M, Mizukami H, Hiraoka N, Konoshima M (1974) Pigment formation in callus cultures of Lithospermumerythrorhizon. Phytochemistry 13(6):927–932
Torgomyan H, Trchounian A (2013) Bactericidal effects of low-intensity extremely high frequency electromagnetic field: an overview with phenomenon, mechanisms, targets and consequences. Crit Rev Microbiol 39(1):102–111
Trchounian A (2004) Escherichia coli proton-translocating FOF1 ATP synthase and its association with solute secondary transporters and/or enzymes of anaerobic oxidation–reduction under fermentation. Biochem Biophys Res Commun 315(4):1051–1057
Trchounian A, Kobayashi H (1998) Relationship of K+-uptaking system with H+-translocating ATPase in Enterococcus hirae, growth at a high or low alkaline pH. Curr Microbiol 36(2):114–118
Vardanyan Z, Trchounian A (2010) The effects of copper (II) ions on Enterococcus hirae cell growth and the proton-translocating FOF1 ATPase activity. Cell Biochem Biophys 57(1):19–26
Vardanyan Z, Trchounian A (2012) Fe(III) and Fe(II) ions different effects on Enterococcus hirae cell growth and membrane-associated ATPase activity. Biochem Biophys Res Commun 417(1):541–545
Xu C, Lin X, Ren H, Zhang Y, Wang S, Peng X (2006) Analysis of outer membrane proteome of Escherichia coli related to resistance to ampicillin and tetracycline. Proteomics 6(2):462–473
Yamamoto H, Yazaki K, Inoue K (2000) Simultaneous analysis of shikimate-derived secondary metabolites in Lithospermum erythrorhizon cell suspension cultures by high-performance liquid chromatography. J Chromatography B Biomed Sci Appl 738(1):3–15
Ye HC, Yin ZH, Li GF, Wu X, Dong JW, Wu ZR (1991) Effect of physical and chemical factors in callus growth and shikonin derivative formation in the callus cultures of Arnebiaeuchroma. Acta Bot Sin 33(12):927–931
Zare k, Nazemiyeh H, Movafeghi A, Khosrowshahli M, Motallebi-Azar A, Dadpour M, Omidi Y (2010) Bioprocess engineering of Echium italicum L.: induction of shikonin and alkannin derivatives by two-liquid-phase suspension cultures. PCTOC J Plant Biotechnol 100(2):157–164
Acknowledgments
This study was done in the frame of Basic research support by State Committee on Science, Ministry of Education and Science of Armenia (#10-3/9).
Conflict of interests
The authors report no declarations of interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Petrosyan, M., Shcherbakova, Y., Sahakyan, N. et al. Alkanna orientalis (L.) Boiss. plant isolated cultures and antimicrobial activity of their extracts: phenomenon, dependence on different factors and effects on some membrane-associated properties of bacteria. Plant Cell Tiss Organ Cult 122, 727–738 (2015). https://doi.org/10.1007/s11240-015-0806-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0806-3