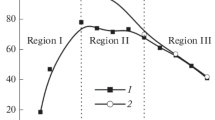
It was shown that the presence of titanium dioxide nanoparticles with an average size of 14 nm in methanesulfonate electrolytes leads to acceleration of the electrolytic deposition and crystallization of lead dioxide. It was established that the process results in the formation of PbO2–TiO2 nanocomposites the composition and structure of which depend on the composition of the suspension electrolyte and the hydrodynamic conditions of electrodeposition.


Similar content being viewed by others
References
O. Shmychkova, T. Luk’yanenko, A. Yakubenko, et al., Appl. Catal. B, 162, 346-351 (2015).
O. Shmychkova, T. Luk’yanenko, A. Velichenko, et al., Electrochim. Acta, 111, 332-338 (2013).
A. B. Velichenko, T. V. Luk’yanenko, N. V. Nikolenko, et al., Russ. J. Electrochem., 43, 118 (2007).
R. Amadelli, L. Samiolo, A. B. Velichenko, et al., Electrochim. Acta, 54, No. 22, 5239-5245 (2009).
X. Li, D. Pletcher, and F. C. Walsh, Chem. Soc. Rev., 40, 3879 (2011).
D. Pletcher, H. Zhou, G. Kear, et al., J. Power Sources, 180, 630-634 (2008).
A. B. Velichenko, R. Amadelli, V. A. Knysh, et al., J. Electroanal. Chem., 632, 192-196 (2009).
A. B. Velichenko, V. A. Knysh, T. V. Luk’yanenko, et al., Russ. J. Appl. Chem., 81, No. 6, 994-999 (2008).
A. B. Velichenko, V. A. Knysh, T. V. Luk’yanenko, et al., Prot. Met. Phys. Chem. Surfaces, 45, No. 3, 327-332 (2009).
A. B. Velichenko, V. A. Knysh, T. V. Luk’yanenko, et al., Mater. Chem. Phys., 131, No. 3, 686-693 (2012).
S. Cattarin and M. Musiani, Electrochim. Acta, 52, No. 4, 1339-1348 (2006).
J. Gonzalez-Garcia, F. Gallud, J. Iniesta, et al., J. Electrochem. Soc., 147, No. 8, 2969-2974 (2000).
M. Y. Abyaneh, V. Saez, J. González-García, and T. J. Mason, Electrochim. Acta, 55, 3572-3579 (2010).
D. Pletcher, Z.-Q. Tian, and D. E. Williams (eds.), Developments in Electrochemistry, Wiley, Chichester (2014), pp. 49-64
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Teoreticheskaya i Éksperimental’naya Khimiya, Vol. 52, No. 2, pp. 125-129, March-April, 2016.
Rights and permissions
About this article
Cite this article
Velichenko, A.B., Knysh, V.A., Luk’yanenko, T.V. et al. Electrodeposition of PbO2–TiO2 Nanocomposite Materials from Suspension Electrolytes. Theor Exp Chem 52, 127–131 (2016). https://doi.org/10.1007/s11237-016-9461-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-016-9461-y