Abstract
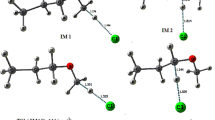
Kinetics and thermochemistry of the H-atom abstraction reaction of CH3OCH2CH2Cl with OH radical have been carried out using dual level of methods. Initially, geometry optimization and frequency calculations are performed at M06-2X/6-31+G(d, p) level of theory, and energetic calculations are further refined using CCSD(T)/6-311++G(d, p) level of theory in order to characterized all stationary points on potential energy surface (PES). The result shows that H-atom abstraction from –OCH2 site of CH3OCH2CH2Cl is dominant path. The rate constants are calculated using canonical transition state theory at 298 K, which are found to be in good agreement with the experimental data. We have presented the standard enthalpies of formation for CH3OCH2CH2Cl and the radicals generated during the H-atom abstraction using group-balanced isodesmic reactions scheme. The atmospheric lifetime of title molecule is also calculated.


Similar content being viewed by others
References
Atkinson R, Arey J (2003) Chem Rev 103:4605–4638
Mellouki A, Le Bras G, Sidebottom H (2003) Chem Rev 103:5077–5096
McClay K, Schaefer CE, Vainberg S, Steffan RJ (2007) Appl Environ Microbiol 73:6870–6875
Coe PL, Rowbotham RA, Tatlow JC (1997) J Fluorine Chem 82:9–12
Sekiya A, Misaki S (2000) J Fluorine Chem 101:215–221
Dalmasso PR, Taccone RA, Nieto JD, Cometto PM, Lane SI (2008) J Phys Org Chem 21:393–396
Dalmasso PR, Taccone RA, Nieto JD, Cometto PM, Lane SI (2005) Int J Chem Kinet 37:420–426
Dalmasso PR, Taccone RA, Nieto JD, Cometto PM, Lane SI (2010) Atmos Environ 44:1749–1753
Dalmasso PR, Taccone RA, Nieto JD, Cometto PM, Lane SI (2012) Atmos Environ 47:104–110
Mishra BK, Chakrabartty AK, Deka RC (2014) Mol Phys 112(11):1512–1519
Mishra BK, Chakrabartty AK, Bhattacharjee D, Deka RC (2014) Struct Chem 24:1621–1626
Hu WP, Truhlar DG (1996) J Am Chem Soc 118:860–869
Truhlar DG, Garrett BC, Klippenstein SJ (1996) J Phys Chem A 100:12771–12800
Zhao Y, Truhlar DG (2008) Theor Chem Acc 120:215–241
Pople JA, Head-Gordon M, Raghavachari K (1987) J Chem Phys 87:5968–5975
Lily M, Mishra BK, Chandra AK (2014) J Fluorine Chem 161:51–59
Dinadayalane TC, Paytakov G, Leszczynski J (2013) J Mol Model 19:2855–2864
Deka RC, Mishra BK (2014) J Mol Graph Model 53:23–30
Sandhiya L, Kolandaivel P, Senthilkumar K (2012) Struct Chem 23:1475–1488
Gour NK, Deka RC, Singh HJ, Mishra BK (2014) J Fluorine Chem 160:64–71
Gonzales C, Schlegel HB (1991) J Chem Phys 95:5853–5860
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V,Mennucci B, Petersson GA, Nakatsuji H, CaricatoM, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, EharaM, ToyotaK, FukudaR, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell K, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09, revision B.01. Gaussian Inc., Wallingford
Laidler KJ (2004) Chemical kinetics, 3rd edn. Pearson Education, New Delhi
Johnston HS, Heicklen JJ (1962) J Phys Chem 66:532–533
Xiao R, Noerpel M, Luk HL, Wei Z, Spinney R (2014) Int J Quantum Chem 114:74–83
Atkins P, de Paula J (2010) Physical chemistry, 9th edn. Oxford University Press, New York
Truhlar DG, Chuang YY (2000) J Chem Phys 112:1221–1228
Hammond GS (1955) J Am Chem Soc 77:334–338
Dalmasso PR, Taccone RA, Nieto JD, Cometto PM, Cobos CJ, Lane SI (2014) Atmos Environ 91:104–109
Chase MW Jr (1989) NIST-JANAF thermochemical tables, 4th edn. J Phys Chem Ref Data Monograph No. 9. ACS & AIP, New York
Csontos J, Rolik Z, Das S, Kallay M (2010) J Phys Chem A 114:13093–13103
Pilcher G, Pell AS, Coleman D (1964) J Trans Faraday Soc 60:499–505
Lide DR (2009) CRC handbook of chemistry and physics, 89th edn. CRC Press, New York
Papadimitriou VC, Kambanis KG, Lazarou YG, Papagiannakopoulos P (2004) J Phys Chem A 108:2666–2674
Atkinson R (1997) J Phys Chem Ref Data 26:215–290
Acknowledgments
NKG is thankful to University Grants Commission (UGC), New Delhi, for providing Dr. D. S. Kothari Postdoctoral Fellowship (Award Letter No: F.4-2/2006(BSR)/CH/14-15/0217). One of the authors IH thanks University Grants Commission for providing the “Maulana Azad National Fellowship” F1-17.1/2013-14/MANF-2013-14-MUS-ASS-25447. The authors also acknowledge financial support from the Department of Science and Technology, New Delhi, in the form of a Project [SR/NM.NS-1023/2011(G)].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gour, N.K., Mishra, B.K., Hussain, I. et al. Theoretical investigation on the kinetics and thermochemisty of H-atom abstraction reactions of 2-chloroethyl methyl ether (CH3OCH2CH2Cl) with OH radical at 298 K. Struct Chem 27, 1491–1499 (2016). https://doi.org/10.1007/s11224-016-0771-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-016-0771-4