Abstract
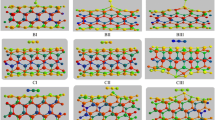
The adsorption behavior of the HCN on the surface of beryllium oxide nanotube (BeONT) is studied by the density functional theory. Geometrical parameters, electronic properties and adsorption energies have been calculated for the BeONT and fourteen different HCN configurations on the nanotube. According to the obtained results, the process of the HCN molecule adsorption on different sites on the external surface of the nanotube is exothermic and all of the configurations are stable, while the process of HCN molecule adsorption on the internal surface of the BeONT is endothermic. The adsorption energy values indicate that the HCN molecule can be physically adsorbed on the surface of the BeONT. Furthermore, the HOMO–LUMO gap (Eg) of the BeONT decreases upon the HCN adsorption, resulting in the enhancement of the electrical conductivity. The AIM theory has been also utilized to analyze the properties of the bond critical points: their electron densities and their Laplacians. NBO analysis indicates that the HCN molecule can be adsorbed on the surface of the nanotube with a charge transfer from nanotube to HCN molecule. Due to the physisorption, NQR parameters of nanotube are also altered. In order to examine the deformation degree of the nanotube after HCN molecule adsorption, deformation energy is calculated, which indicates that no significant curvature in the geometry of the nanotubes is occurred when HCN adsorbs onto the surface of BeONT.







Similar content being viewed by others
References
Wang R, Zhang D (2008) Theoretical study of the adsorption of carbon monoxide on pristine and silicon-doped boron nitride nanotubes. Aust J Chem 61:941–946
Ahmadi Peyghan A, Omidvar A, Hadipour NL, Bagheri Z, Kamfiroozi M (2012) Can aluminum nitride nanotubes detect the toxic NH3 molecules? Physica E 44:1357–1360
Kong J, Franklin NR, Zhou C, Chapline MG, Peng S, Cho K, Dai H (2000) Nanotube molecular wires as chemical sensors. Scie. 287:622–625
Collins PG, Bradley K, Ishigami M, Zettl A (2000) Extreme oxygen sensitivity of electronic properties of carbon nanotubes. Science 287:1801–1804
Raissi H, Mollania F (2014) Immunosuppressive agent leflunomide: a SWNTs-immobilized dihydroortate dehydrogenase inhibitory effect and computational study of its adsorption properties on zigzag single walled (6,0) carbon and boron nitride nanotubes as controlled drug delivery devices. Eur J Pharm Sci 56:37–54
Valentini L, Armentano I, Kenny JM, Cantalini C, Lozzi L, Santucci S (2003) Sensors for sub-ppm NO2 gas detection based on carbon nanotube thin films. Appl Phys Lett 82:961–963
Zurek B, Autschbach J (2004) Density functional calculations of the 13C NMR chemical shifts in (9,0) single-walled carbon nanotubes. J Am Chem Soc 126:13079–13088
Duman S, Sütlü A, Bagci S, Tütüncü HM, Srivastava GP (2009) Structural, elastic, electronic, and phonon properties of zinc-blende and wurtzite BeO. J Appl Phys 105:033719-1–033719-8
Sorokin PB, Fedorov AS, Chernozatonskii LA (2006) Structure and properties of BeO nanotubes. Phys Solid State 48:398–401
Baumeier B, Kruger P, Pollmann J (2007) Structural, elastic, and electronic properties of SiC, BN, and BeO nanotubes. Phys Rev B 76:085407(1)–085407(10)
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Boys SB, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566
Koopmans TA (1934) Ordering of wave functions and eigenenergies to the individual electrons of an atom. Physica 1:104–113
Pearson RG (1985) Absolute electronegativity and absolute hardness of Lewis acids and bases. J Am Chem Soc 107:6801–6806
Parr RG, Chattaraj PK (1991) Aspects of softness and hardness … Principle of maximum hardness. J Am Chem Soc 113:1854–1855
Biegler- König F, Schönbohm J (2002) Update of the AIM2000-program for atoms in molecules. J Comput Chem 23:1489–1494
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor–acceptor viewpoint. Chem Rev 88:899–926
Glendening ED, Reed AE, Carpenter JE, Weinhold F (1992) NBO Version 3.1. Gaussian, Inc., Pittsburgh
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JJA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al- Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision C. 02. Gaussian, Inc., Wallingford
Ehlers AW, Baerends EJ, Lammertsma K (2002) Nucleophilic or electrophilic phosphinidene complexes ML(n)=PH; what makes the difference? J Am Chem Soc 124:2831–2838
Pandey KK, Frenking G (2004) The nature of the M≡E bond: Theoretical investigation of the molecules [(OR)3M≡E] (M=Mo, W; E=N, P, As, Sb, Bi; R=H, Me) and [(Me3CO)3Mo≡P]. Eur J Inorg Chem 2004:4388–4395
Pyykkö P (2001) Spectroscopic nuclear quadrupole moments. Mol Phys 99:1617–1629
Lucken EAC (1992) Nuclear quadrupole coupling constants. Academic Press, London
Sarmah A, Saha S, Bagaria P, Roy RK (2011) On the complementarity of comprehensive decomposition analysis of stabilization energy (CDASE)–Scheme and supermolecular approach. Chem Phys 394:29–35
Cyran´ski MK, Krygowski TM, Katritzky AR, Schleyer PvR (2002) To what extent can aromaticity be defined uniquely? J Org Chem 67:1333–1338
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marvi, M., Raissi, H. & Ghiassi, H. Effects of the HCN adsorption on the structural and electronic parameters of the beryllium oxide nanotube. Struct Chem 27, 557–571 (2016). https://doi.org/10.1007/s11224-015-0585-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-015-0585-9