Abstract
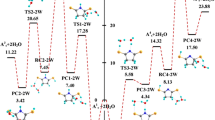
Isomerizations and tautomerisms in succinimide were studied using B3LYP and MP2 methods. Activation energies (E a) and Gibbs free energies of activation (∆G #) were calculated for O–H rotations, keto–enol, and amine–imine tautomerisms. The calculated activation energies for the O–H rotations were generally smaller than 30 kJ/mol and for keto → enol and amine → imine tautomerisms were about 300 and 210 kJ/mol, respectively. Water molecules were used to catalyze the proton transfer reactions in the keto–enol and amine–imine tautomerisms. It was found that water molecules decrease the activation energies of the tautomerisms. Also, catalytic effect of two water molecules was more than that of one water molecule.



Similar content being viewed by others
References
Patsalos PN (2005) Properties of antiepileptic drugs in the treatment of idiopathic generalized epilepsies. Epilepsia 46:140–148
Fujiwara K, Matsumoto N, Kitagawa T, Inouye K (1990) The use of N-(aminobenzoyloxy) succinimide as a two-level heterobifunctional agent for the preparation of hapten-protein conjugateds. Daunomycin as a model hapten with an amino group. J Immunol Methods 134:227–235
Shen JX, Brodbelt J (1996) Reactions of Hydantoin and succinimide anticonvulsants with dimethyl ether ions in a Quadrupole ion trap Mass Spectrometer. J Mass Spectrom 31:1389–1398
Capasso S, Mazzarella L, Sica F, Zagari A, Salvadori S (1993) Kinetics and mechanism of succinimide ring formation in the deamidation process of asparagine residues. J Chem Soc Perkin Trans 2:679–682
Kalia S, Sharma A, Kaith BS (2007) Ab initio study of gas phase and water assisted tautomerization of maleimide and formamide. J Chem Sci 119:617–624
Kaliman I, Nemukhin A, Varfolomeev S (2010) Free energy barriers for the N-terminal asparagine to succinimide conversion: quantum molecular dynamics simulations for the fully solvated model. J Chem Theory Comput 6:184–189
Takahashi O, Kobayashi K, Oda A (2010) Modeling the enolization of succinimide derivatives, a key step of racemization of aspartic acid residues: importance of a two-H2O mechanism. Chem Biodivers 7:1349–1356
Konuklar FA, Aviyente V, Lopez MFR (2002) Theoretical study on the alkaline and neutral hydrolysis of succinimide derivatives in deamidation reactions. J Phys Chem 106:11205–11214
Valadbeigi Y, Farrokhpour H, Tabrizchi M (2014) DFT study on the isomerization in vitamin B6. Struct Chem. doi:10.1007/s11224-014-0402-x
Chahkandi B, Tayyari SF, Bakhshaei M, Chahkandi M (2013) Investigation of simple and water assisted tautomerism in a derivative of 1,3,4-oxadiazole: a DFT study. J Mol Graph Model 44:120–128
Li Q-G, Xue Y, Yan G-S (2008) Water-assisted enol-to-keto tautomerism of simple peptide model: a computational investigation. J Mol Struct 868:55–64
Valadbeigi Y, Farrokhpour H (2014) Intramolecular H-transfer in CH2OO and cis-HO3. Struct Chem. doi:10.1007/s11224-014-0455-x
Vohringer-Martinez E, Toro-Labbe A (2010) The role of water in the proton transfer reaction mechanism in tryphtophan. J Comput Chem 31:2642–2649
Fukui K (1981) The path of chemical reactions—the IRC approach. Acc Chem Res 14:363–368
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr., Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.1. Gaussian, Inc., Wallingford
Perez P, Contreras R (1998) A theoretical analysis of the gas-phase protonation of hydroxylamines, methyl-derivatives and aliphatic amino acids. Chem Phys Lett 293:239–244
Acknowledgments
The authors wish to express thanks to the Center of Excellency in Chemistry of Isfahan University of Technology.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Valadbeigi, Y., Farrokhpour, H. Simple and water-assisted tautomerism in succinimide. Struct Chem 26, 539–545 (2015). https://doi.org/10.1007/s11224-014-0511-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-014-0511-6