Abstract
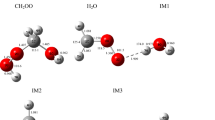
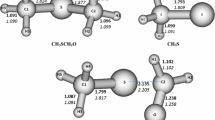
The isomerization of CH2SH to CH3S in the absence and presence of atmospheric nucleation precursors (H2O, HCOOH, and H2SO4) has been investigated at the G3XMP2//B3LYP/6–311+G(3df,2p) level. It is shown that the barrier heights for the isomerization are 28.74, 6.45, 12.96, and 19.23 kcal mol−1, when the isomerization is performed in gas phase and with sulfuric acid, water, and formic acid as a precursor, respectively. The rate constants of the isomerization are calculated using the transition state theory with the Wigner tunneling correction over the temperature range of 298–800 K. The rate constant of sulfuric acid-catalyzed isomerization is 1.46 × 103 times at 298 K larger than that in gas phase. Sulfuric acid-catalyzed isomerization is expected to be favorable under atmospheric condition. Moreover, topology analysis has been carried out to characterize the nature of interactions in the isomerization. Enthalpies of formation (∆f H °298K ), entropies (S °298K ), and heat capacities (C p) of all the stabilized species have been calculated using CBS-QB3 method. These results can be used for atmosphere sulfur cycle modeling application.





Similar content being viewed by others
References
Barnes I, Hjorth J, Mihalopoulos N (2006) Chem Rev 106:940–975
Aranda A, Mera YD, Rodríguez D, Salgado S, Martínez E (2002) Chem Phys Lett 357:471–476
Cardoso DV, Ferrão LFA, Keidel Spada RF, Roberto-Neto O, Correto Machado FB (2012) Int J Quantum Chem 112:3269–3275
Green M, Lown EM, Strausz OP (1984) J Am Chem Soc 106:6938–6946
Vandeputte AG, Reyniers MF, Marin GB (2010) J Phys Chem A 114:10531–10549
Tsai IC, Chen JP, Lin PY, Wang WC, Isaksen ISA (2010) Atmos Chem Phys 10:3693–3709
Zhan PY, Pan YR, Tang YZ (2009) Chem Phys 360:53–58
Li XY, Zeng YL, Ling MP, Zheng SJ (2005) Acta Chim Sinica 63:352–357
Pei KM, Li YM, Kong XL, Li HY (2003) Chin J Chem Phys 16:251–256
Wang WL, Liu Y, Wang WN, Luo Q, Li QS (2005) Acta Chim Sinica 17:1554–1560
Wang TF, Bowie JH (2012) Org Biomol Chem 10:3219–3228
Jørgensen S, Jensen C, Kjaergaard HG, Anglada JM (2013) Phys Chem Chem Phys 15:5140–5150
Elm J, Bilde M, Mikkelsen KV (2013) J Phys Chem A 117:6695–6701
Buszek RJ, Sinha A, Francisco JS (2011) J Am Chem Soc 133:2013–2015
Gonzalez J, Anglada JM, Buszek RJ (2011) J Am Chem Soc 133:3345–3353
Hazra MK, Francisco JS, Sinha A (2013) J Phys Chem A 117:11704–11710
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Curtiss LA, Redfern PC, Raghavachari K, Pople JA (2001) J Chem Phys 114:108–117
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, J. A. Montgomery J, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2003) Gaussian 03, Revision C.02. Gaussian, Inc., WallingfordFrisch
Duncan WT, Bell RL, Truong TN (1998) J Comput Chem 19:1039–1052
Zhang SW, Truong TN (2001) VKLab version 1.0, University of Utah
Montgomery JA, Frisch MJ, Ochterski JW, Petersson GA (1999) J Chem Phys 110:2822–2827
Curtiss LA, Raghavachari K, Redfern PC, Pople JA (1997) J Chem Phys 106:1063–1079
Barker JR, Ortiz F, Lawrence JMP, et al (2010) MultiWell-2010 Software
Bader RFW (1994) Atoms in Molecules A Quantum Theory. Oxford University Press, New York
Gonzalez J, Torrent-Sucarrat M, Anglada JM (2010) Phys Chem Chem Phys 12:2116–2125
Biswal HS, Shirhatti PR, Wategaonkar S (2009) J Phys Chem A 113:5633–5643
Lu T, Chen WF (2012) J Comput Chem 33:580–592
Lu T, Chen FW (2012) J Mol Graph Model 38:314–323
Russell D, Johnson III (2013) NIST Standard Reference Database Number 101, Release 16a. http://cccbdb.nist.gov/Intro.asp. accessed May 20, 2014
Liu Y, Wang WL, Wang WN, Su KH, Zhang Y (2008) J Mol Struct 866:46–51
Nguyen VS, Orlando TM, Leszczynski J, Nguyen MT (2013) J Phys Chem A 117:2543–2555
Li P, Niu WX, Gao T, Wang HY (2014) Int J Quantum Chem 114:760–768
Mandal D, Bagchi S, Das AK (2012) Chem Phys Lett 551:31–37
Vandeputte AG, Reyniers MF, Marin GB (2009) Theor Chem Acc 123:391–412
Hahn DK, Raghu Veer KS, Ortiz JV (2010) J Phys Chem A 114:8142–8155
Nagy B, Szakács P, Csontos J, Rolik Z, Tasi G, Kállay M (2011) J Phys Chem A 115:7823–7833
Saebø S, Radom L, Schaefer HF III (1983) J Chem Phys 78:845–853
Acknowledgments
We thank the School of Chemistry & Chemical engineering of Shaanxi Normal University for access to the high performance computing service. This work is supported by the foundation of Shaanxi Education Department (2013JK0667), the foundation of Yan’an University (YDQ2013-16), and the foundation of College of Chemistry & Chemical engineering of Yan’an university (YDHG2014-Z04).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11224_2014_489_MOESM1_ESM.doc
Supplementary material 1 (DOC 672 kb) Appendix A. Supplementary data Optimized geometries of all the species at the B3LYP/6-311+G(3df,2p) level are given in Fig. S1. The molecular graphs of the transition states and intermediates calculated using B3LYP/6-311+G(3df,2p) method are shown in Fig. S2. The zero point energies (ZPE) and electronic energies (E) of all the species are listed in Table S1. The predicted ratios of rate over the temperature range of 298~800 K are listed in Table S2.
Rights and permissions
About this article
Cite this article
Cao, J., Wang, Z.X., Gao, L.J. et al. Atmospheric nucleation precursors catalyzed isomerization of CH2SH to CH3S: mechanisms and topological analysis. Struct Chem 26, 261–268 (2015). https://doi.org/10.1007/s11224-014-0489-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-014-0489-0