Abstract
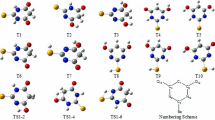
Isomerization and tautomerism reactions of the active form of vitamin B6, pyridoxal phosphate, are studied at B3LYP level of theory using 6-311++G(2df,p) basis set in gas and aqueous phases. Twenty-three transition state (TS) structures for vitamin B6 isomerization are optimized, including 13 TS structures for O–H and C–C rotations, 8 TS structures for imine–enamine tautomerism, and 2 TS structures for keto–enol tautomerism. Activation energy (E a), imaginary frequency (υ), and Gibbs free energy of activation (ΔG #) for the isomerization reactions are calculated. The activation energies of the imine–enamine tautomerism are in the range of 190–280 kJ/mol and of O–H and C–C rotations are mainly less than 60 kJ/mol. Also, our calculation shows that the imine forms of B6 are mainly more stable than the enamine forms. Effect of microhydration on the TS structures and activation energies is also investigated. It is found that the presence of water molecules catalyzes only the imine–enamine tautomerism.





Similar content being viewed by others
References
Schneider G, Kack H, Lindqvist Y (2000) The manifold of vitamin B6 dependent enzymes. Structure 8:R1–R6
Combs GF (2008) The vitamins: fundamental aspects in nutrition and health, 3rd edn. Elsevier Academic Press, San Diego
Lichtstein HC, Gunsalus IC, Umbreit WW (1945) Function of the vitamin B6 group: pyridoxal phosphate (codecarboxylase) in transamination. J Biol Chem 161:311–320
Harris SA, Webb TJ, Folkers K (1940) Chemistry of vitamin B6. I. Tautomerism. J Am Chem Soc 62:3198–3203
Mahfouz MM, Zhou SQ, Kummerow FA (2009) Vitamin B6 compounds are capable of reducing the superoxide radical and lipid peroxide levels induced by H2O2 in vascular endothelial cells in culture. Int J Vitam Nutr Res 79:218–229
Kannan K, Jain SK (2004) Effect of vitamin B6 on oxygen radicals, mitochondrial membrane potential, and lipid peroxidation in H2O2-treated U937 monocytes. Free Radic Biol Med 36:423–428
Raczynska ED, Duczmal K, Darowska M (2005) Experimental (FT-IR) and theoretical (DFT-IR) studies of keto–enol tautomerism in pyruvic acid. Vib Spectr 39:37–45
Carpy AJM, Haasbroek PP, Ouhabi J, Oliver DW (2000) Keto/enol tautomerism in phenylpyruvic acids: structure of the o-nitrophenylpyruvic acid. J Mol Struct: Theochem 520:191–198
Huang Y, Zhang X, Xu L, Chen H, Chen G (2009) Characterization of keto–enol tautomerism of p-hydroxyphenylpyruvic acid using CE with amperometric detection and spectrometric analysis. J Sep Sci 32:4155–4160
Valadbeigi Y, Farrokhpour H (2013) Theoretical study on keto–enol tautomerism and isomerization in pyruvic acid. Int J Quant Chem 113:2372–2378
Fu A-P, Li H-L, Du D-M, Zhou Z-Y (2003) Theoretical study on the reaction mechanism of proton transfer in formamide. Chem Phys Lett 382:332–337
Matxain JM, Ristilä M, Strid A, Eriksson LA (2007) Theoretical study of the reaction of vitamin B6 with 1O2. Chem Eur J 13:4636–4642
Kiruba GS, Wong MW (2003) Tautomeric equilibria of pyridoxal-5′-phosphate (vitamin B6) and 3-hydroxypyridine derivatives: a theoretical study of solvation effects. J Org Chem 68:2874–2881
Sahoo SK, Sharma D, Bera RK (2012) Studies on molecular structure and tautomerism of a vitamin B6 analog with density functional theory. J Mol Model 5:1993–2001
Wei DQ, Proynov EI, Milet A, Salahub DR (2000) Solvation of the hydroxide anion: a combined DFT and molecular dynamics study. J Phys Chem 104:2384–2395
Miertus S, Tomasi J (1982) Approximate evaluations of the electrostatic free energy and internal energy changes in solution processes. Chem Phys 65:239–245
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision A.1. Gaussian, Inc., Wallingford
Alvarez-Idaboy JR, Mora-Diez N, Vivier-Bunge A (2000) A quantum chemical and classical transition state theory. Explanation of negative activation energies in OH addition to substituted ethenes. J Am Chem Soc 122:3715–3720
Charmchini AN, Farrokhpour H, Teimouri A, Pourmoghaddas F (2013) Theoretical studies on tautomerism of imidazole-2-selenone. Struct Chem 24:1215–1227
Markova N, Enchev V, Timitcheva I (2005) Oxo-hydroxy tautomerism of 5-fluorouracil: water-assisted proton transfer. J Phys Chem A 109:1981–1988
Kyrychenko A, Waluk J (2006) Excited-state proton transfer through water bridges and structure of hydrogen-bonded complexes in 1H-pyrrolo[3,2-h]quinoline: adiabatic time-dependent density functional theory study. J Phys Chem A 110:11958–11967
Acknowledgments
The authors wish to express thanks to the Center of Excellency in Chemistry of Isfahan University of Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valadbeigi, Y., Farrokhpour, H. & Tabrizchi, M. DFT study on the isomerization in vitamin B6. Struct Chem 25, 1395–1404 (2014). https://doi.org/10.1007/s11224-014-0402-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-014-0402-x